Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes
- PMID: 15322084
- PMCID: PMC1201506
- DOI: 10.1074/jbc.M407986200
Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes
Abstract
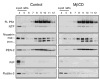
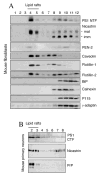
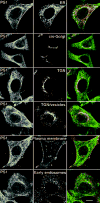
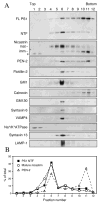
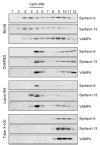
Alzheimer's disease-associated beta-amyloid peptides (Abeta) are generated by the sequential proteolytic processing of amyloid precursor protein (APP) by beta- and gamma-secretases. There is growing evidence that cholesterol- and sphingolipid-rich membrane microdomains are involved in regulating trafficking and processing of APP. BACE1, the major beta-secretase in neurons is a palmitoylated transmembrane protein that resides in lipid rafts. A subset of APP is subject to amyloidogenic processing by BACE1 in lipid rafts, and this process depends on the integrity of lipid rafts. Here we describe the association of all four components of the gamma-secretase complex, namely presenilin 1 (PS1)-derived fragments, mature nicastrin, APH-1, and PEN-2, with cholesterol-rich detergent insoluble membrane (DIM) domains of non-neuronal cells and neurons that fulfill the criteria of lipid rafts. In PS1(-/-)/PS2(-/-) and NCT(-/-) fibroblasts, gamma-secretase components that still remain fail to become detergent-resistant, suggesting that raft association requires gamma-secretase complex assembly. Biochemical evidence shows that subunits of the gamma-secretase complex and three TGN/endosome-resident SNAREs cofractionate in sucrose density gradients, and show similar solubility or insolubility characteristics in distinct non-ionic and zwitterionic detergents, indicative of their co-residence in membrane microdomains with similar protein-lipid composition. This notion is confirmed using magnetic immunoisolation of PS1- or syntaxin 6-positive membrane patches from a mixture of membranes with similar buoyant densities following Lubrol WX extraction or sonication, and gradient centrifugation. These findings are consistent with the localization of gamma-secretase in lipid raft microdomains of post-Golgi and endosomes, organelles previously implicated in amyloidogenic processing of APP.
Figures



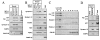
Similar articles
-
Spatial segregation of gamma-secretase and substrates in distinct membrane domains.J Biol Chem. 2005 Jul 8;280(27):25892-900. doi: 10.1074/jbc.M503570200. Epub 2005 May 10. J Biol Chem. 2005. PMID: 15886206 Free PMC article.
-
S-palmitoylation of gamma-secretase subunits nicastrin and APH-1.J Biol Chem. 2009 Jan 16;284(3):1373-84. doi: 10.1074/jbc.M806380200. Epub 2008 Nov 20. J Biol Chem. 2009. PMID: 19028695 Free PMC article.
-
Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts.Curr Biol. 2001 Aug 21;11(16):1288-93. doi: 10.1016/s0960-9822(01)00394-3. Curr Biol. 2001. PMID: 11525745
-
Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex.Neuron. 2003 Apr 10;38(1):9-12. doi: 10.1016/s0896-6273(03)00205-8. Neuron. 2003. PMID: 12691659 Review.
-
Mechanisms of disease: new therapeutic strategies for Alzheimer's disease--targeting APP processing in lipid rafts.Nat Clin Pract Neurol. 2007 Jul;3(7):374-82. doi: 10.1038/ncpneuro0549. Nat Clin Pract Neurol. 2007. PMID: 17611486 Free PMC article. Review.
Cited by
-
Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer's disease.Aging Cell. 2012 Aug;11(4):559-68. doi: 10.1111/j.1474-9726.2012.00817.x. Epub 2012 Apr 9. Aging Cell. 2012. PMID: 22404891 Free PMC article.
-
Functional expression and microdomain localization of prestin in cultured cells.Otolaryngol Head Neck Surg. 2007 Mar;136(3):434-9. doi: 10.1016/j.otohns.2006.10.030. Otolaryngol Head Neck Surg. 2007. PMID: 17321873 Free PMC article.
-
Dependency of γ-secretase complex activity on the structural integrity of the bilayer.Biochem Biophys Res Commun. 2010 Nov 12;402(2):291-6. doi: 10.1016/j.bbrc.2010.10.017. Epub 2010 Oct 16. Biochem Biophys Res Commun. 2010. PMID: 20937251 Free PMC article.
-
Identification of novel acetylcholinesterase inhibitors designed by pharmacophore-based virtual screening, molecular docking and bioassay.Sci Rep. 2018 Oct 8;8(1):14921. doi: 10.1038/s41598-018-33354-6. Sci Rep. 2018. PMID: 30297729 Free PMC article.
-
The Potential Therapeutic Application of Simvastatin for Brain Complications and Mechanisms of Action.Pharmaceuticals (Basel). 2023 Jun 22;16(7):914. doi: 10.3390/ph16070914. Pharmaceuticals (Basel). 2023. PMID: 37513826 Free PMC article. Review.
References
-
- Selkoe DJ. Physiol Rev. 2001;81:741–766. - PubMed
-
- Sisodia SS, St George-Hyslop PH. Nat Rev Neurosci. 2002;3:281–290. - PubMed
-
- Vassar R. J Mol Neurosci. 2004;23:105–114. - PubMed
-
- Iwatsubo T. Curr Opin Neurobiol. 2004;14:379–383. - PubMed
-
- Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Annu Rev Genet. 1998;32:461–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

