Insulin resistance in skeletal muscles of caveolin-3-null mice
- PMID: 15314230
- PMCID: PMC515114
- DOI: 10.1073/pnas.0402053101
Insulin resistance in skeletal muscles of caveolin-3-null mice
Abstract
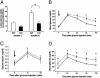
Type 2 diabetes is preceded by the development of insulin resistance, in which the action of insulin is impaired, largely in skeletal muscles. Caveolin-3 (Cav3) is a muscle-specific subtype of caveolin, an example of a scaffolding protein found within membranes. Cav is also known as growth signal inhibitor, although it was recently demonstrated that the genetic disruption of Cav3 did not augment growth in mice. We found, however, that the lack of Cav3 led to the development of insulin resistance, as exemplified by decreased glucose uptake in skeletal muscles, impaired glucose tolerance test performance, and increases in serum lipids. Such impairments were markedly augmented in the presence of streptozotocin, a pancreatic beta cell toxin, suggesting that the mice were susceptible to severe diabetes in the presence of an additional risk factor. Insulin-stimulated activation of insulin receptors and downstream molecules, such as IRS-1 and Akt, was attenuated in the skeletal muscles of Cav3 null mice, but not in the liver, without affecting protein expression or subcellular localization. Genetic transfer of Cav3 by needle injection restored insulin signaling in skeletal muscles. Our findings suggest that Cav3 is an enhancer of insulin signaling in skeletal muscles but does not act as a scaffolding molecule for insulin receptors.
Figures


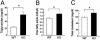
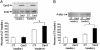
Similar articles
-
Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle.Am J Physiol Cell Physiol. 2005 Jun;288(6):C1317-31. doi: 10.1152/ajpcell.00489.2004. Epub 2005 Feb 2. Am J Physiol Cell Physiol. 2005. PMID: 15689413
-
Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue.Am J Physiol Cell Physiol. 2003 Jul;285(1):C222-35. doi: 10.1152/ajpcell.00006.2003. Epub 2003 Mar 26. Am J Physiol Cell Physiol. 2003. PMID: 12660144
-
Regulation of insulin response in skeletal muscle cell by caveolin status.J Cell Biochem. 2006 Oct 15;99(3):747-58. doi: 10.1002/jcb.20943. J Cell Biochem. 2006. PMID: 16676355
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
Role of caveolin and caveolae in insulin signaling and diabetes.Am J Physiol Endocrinol Metab. 2003 Dec;285(6):E1151-60. doi: 10.1152/ajpendo.00324.2003. Am J Physiol Endocrinol Metab. 2003. PMID: 14607781 Review.
Cited by
-
Heterozygous caveolin-3 mice show increased susceptibility to palmitate-induced insulin resistance.Physiol Rep. 2016 Mar;4(6):e12736. doi: 10.14814/phy2.12736. Epub 2016 Mar 31. Physiol Rep. 2016. PMID: 27033451 Free PMC article.
-
Dual function of MG53 in membrane repair and insulin signaling.BMB Rep. 2016 Aug;49(8):414-23. doi: 10.5483/bmbrep.2016.49.8.079. BMB Rep. 2016. PMID: 27174502 Free PMC article. Review.
-
Electrical stimulated GLUT4 signalling attenuates critical illness-associated muscle wasting.J Cachexia Sarcopenia Muscle. 2022 Aug;13(4):2162-2174. doi: 10.1002/jcsm.12978. Epub 2022 May 3. J Cachexia Sarcopenia Muscle. 2022. PMID: 35502572 Free PMC article.
-
Metabolic regulation through the endosomal system.Traffic. 2019 Aug;20(8):552-570. doi: 10.1111/tra.12670. Epub 2019 Jun 24. Traffic. 2019. PMID: 31177593 Free PMC article. Review.
-
Insulin Antagonizes LPS-Induced Inflammatory Responses by Activating SR-A1/ERK Axis in Macrophages.Inflammation. 2019 Apr;42(2):754-762. doi: 10.1007/s10753-018-0933-1. Inflammation. 2019. PMID: 30488142
References
-
- Koistinen, H. A. & Zierath, J. R. (2002) Ann. Med. 34, 410-418. - PubMed
-
- Zierath, J. R. & Wallberg-Henriksson, H. (2002) Ann. N.Y. Acad. Sci. 967, 120-134. - PubMed
-
- Couet, J., Sargiacomo, M. & Lisanti, M. P. (1997) J. Biol. Chem. 272, 30429-30438. - PubMed
-
- Yamamoto, M., Toya, Y., Jensen, R. A. & Ishikawa, Y. (1999) Exp. Cell Res. 247, 380-388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

