TLR9-/- and TLR9+/+ mice display similar immune responses to a DNA vaccine
- PMID: 15312142
- PMCID: PMC1782555
- DOI: 10.1111/j.1365-2567.2004.01938.x
TLR9-/- and TLR9+/+ mice display similar immune responses to a DNA vaccine
Abstract
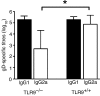
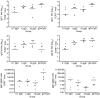
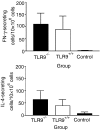
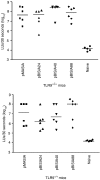
Plasmid DNA continues to attract interest as a potential vaccine-delivery vehicle. However, the mechanisms whereby immune responses are elicited by plasmids are not fully understood. Although there have been suggestions regarding the importance of CpG motifs in plasmid immunogenicity, the molecular mechanisms by which CpG motifs enhance immune responses to DNA vaccines are not well understood. As Toll-like receptor 9-deficient (TLR9-/-) mice fail to respond to the adjuvant effects of CpG oligonucleotides, we used these mice to determine the effect of CpG motifs in plasmids used for DNA immunization. In the study described below, we report that DNA immunization was as effective in eliciting antigen-specific antibody and at stimulating antigen-specific interferon-gamma (IFN-gamma)-secreting cells in TLR9-/- mice as in TLR9+/+ mice. This study illustrates that DNA vaccines elicit immune responses by multiple mechanisms and demonstrates that TLR9 is not essential for the induction of immune responses following DNA immunization.
Figures

Similar articles
-
TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines.Vaccine. 2005 Jan 26;23(10):1258-64. doi: 10.1016/j.vaccine.2004.09.001. Vaccine. 2005. PMID: 15652668
-
Priming of CD8+ T-cell responses after DNA immunization is impaired in TLR9- and MyD88-deficient mice.Vaccine. 2007 Aug 21;25(34):6341-7. doi: 10.1016/j.vaccine.2007.06.016. Epub 2007 Jun 29. Vaccine. 2007. PMID: 17628235
-
CpG immuno-stimulatory motifs enhance humoral immune responses against hepatitis C virus core protein after DNA-based immunization.Arch Virol. 2003 Mar;148(3):435-48. doi: 10.1007/s00705-002-0935-y. Arch Virol. 2003. PMID: 12607097
-
In vitro assay of immunostimulatory activities of plasmid vectors.Methods Mol Med. 2006;127:55-70. doi: 10.1385/1-59745-168-1:55. Methods Mol Med. 2006. PMID: 16988446 Review.
-
TLR9 agonists as adjuvants for prophylactic and therapeutic vaccines.Curr Opin Mol Ther. 2007 Feb;9(1):45-52. Curr Opin Mol Ther. 2007. PMID: 17330401 Review.
Cited by
-
NF-κB activation during intradermal DNA vaccination is essential for eliciting tumor protective antigen-specific CTL responses.Hum Vaccin Immunother. 2013 Oct;9(10):2189-95. doi: 10.4161/hv.25699. Epub 2013 Jul 24. Hum Vaccin Immunother. 2013. PMID: 23884215 Free PMC article.
-
Essential role for TLR9 in prime but not prime-boost plasmid DNA vaccination to activate dendritic cells and protect from lethal viral infection.J Immunol. 2010 Jun 15;184(12):7100-7. doi: 10.4049/jimmunol.0803935. Epub 2010 May 7. J Immunol. 2010. PMID: 20483769 Free PMC article.
-
Regional infusion of a class C TLR9 agonist enhances liver tumor microenvironment reprogramming and MDSC reduction to improve responsiveness to systemic checkpoint inhibition.Cancer Gene Ther. 2022 Dec;29(12):1854-1865. doi: 10.1038/s41417-022-00484-z. Epub 2022 Jun 14. Cancer Gene Ther. 2022. PMID: 35697801 Free PMC article.
-
Identification of Aim2 as a sensor for DNA vaccines.J Immunol. 2015 Jan 15;194(2):630-6. doi: 10.4049/jimmunol.1402530. Epub 2014 Dec 8. J Immunol. 2015. PMID: 25488991 Free PMC article.
-
The future of human DNA vaccines.J Biotechnol. 2012 Dec 31;162(2-3):171-82. doi: 10.1016/j.jbiotec.2012.08.012. Epub 2012 Sep 7. J Biotechnol. 2012. PMID: 22981627 Free PMC article. Review.
References
-
- Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. - PubMed
-
- Manickan E, Yu Z, Rouse RJ, Wire WS, Rouse BT. Induction of protective immunity against herpes simplex virus with DNA encoding the immediate early protein, ICP 27. Viral Immunol. 1995;8:53–61. - PubMed
-
- MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. - PubMed
-
- Ugen KE, Nyland SB, Boyer JD, et al. DNA vaccination with HIV-1 expressing constructs elicits immune responses in humans. Vaccine. 1998;16:1818–21. - PubMed
-
- van Drunen Littel-van den Hurk S, Gerdts V, Loehr BI, Pontarollo R, Rankin R, Uwiera R, Babiuk LA. Recent advances in the use of DNA vaccines for the treatment of diseases of farmed animals. Adv Drug Deliv Rev. 2000;43:13–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
