First clinical trials of a new heteropolymer technology agent in normal healthy volunteers and patients with systemic lupus erythematosus: safety and proof of principle of the antigen-heteropolymer ETI-104
- PMID: 15308520
- PMCID: PMC1755118
- DOI: 10.1136/ard.2003.016691
First clinical trials of a new heteropolymer technology agent in normal healthy volunteers and patients with systemic lupus erythematosus: safety and proof of principle of the antigen-heteropolymer ETI-104
Abstract
Background: The heteropolymer technology was developed to remove pathogens from the circulation.
Objectives: To evaluate the safety and tolerability of a single administration and to establish proof of principle for ETI-104 in normal healthy volunteers (NHV) and patients with systemic lupus erythematosus (SLE) METHODS: The drug was given intravenously to 11 NHV and six patients with SLE. Over 28 days, vital signs were noted, a haematological and chemical analysis of blood and urine was carried out, and adverse events were recorded. CR1 receptor numbers, the ability of antigen based heteropolymers to bind to red blood cells (RBCs), and the clearance of high avidity and total anti-dsDNA antibodies were measured by Farr assays and FACS analysis.
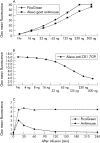
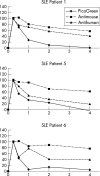
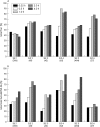
Results: No safety measure differed significantly from normal in both groups; no drug related serious adverse events occurred. ETI-104 rapidly bound to RBCs in NHV and patients with SLE. Binding of the drug to RBCs of patients with SLE also caused a rapid reduction of circulating anti-dsDNA antibodies in the plasma 15 minutes after administration, with a maximum reduction of 55% (range 43-62). At 28 days statistically significant decreases were maintained in three patients, while in the other three the values had returned to baseline levels.
Conclusion: These clinical trials established the safety and the proof of principle of the new immunoconjugate ETI-104. This provides the basis for further development of this technology for numerous indications-for example, therapeutic options for autoimmune diseases or viral and bacterial infections.
Figures

Similar articles
-
BioPlex 2200 multiplexed system: simultaneous detection of anti-dsDNA and anti-chromatin antibodies in patients with systemic lupus erythematosus.Autoimmunity. 2009 Jan;42(1):63-8. doi: 10.1080/08916930802354906. Autoimmunity. 2009. PMID: 19127456
-
Anti-telomere antibodies in systemic lupus erythematosus (SLE): a comparison with five antinuclear antibody assays in 430 patients with SLE and other rheumatic diseases.Ann Rheum Dis. 2004 Oct;63(10):1250-4. doi: 10.1136/ard.2003.011890. Ann Rheum Dis. 2004. PMID: 15361381 Free PMC article.
-
Clearance of anti-double-stranded DNA antibodies: the natural immune complex clearance mechanism.Arthritis Rheum. 2000 Oct;43(10):2265-75. doi: 10.1002/1529-0131(200010)43:10<2265::AID-ANR14>3.0.CO;2-J. Arthritis Rheum. 2000. PMID: 11037886
-
Evaluation of antigen-based heteropolymer for treatment of systemic lupus erythematosus in a nonhuman primate model.Clin Immunol. 2002 Nov;105(2):141-54. doi: 10.1006/clim.2002.5274. Clin Immunol. 2002. PMID: 12482388
-
[The clinical immunology laboratory in diagnosis and monitoring of systemic lupus erythematosus and connective tissue diseases].G Ital Nefrol. 2005 Nov-Dec;22 Suppl 33:S21-6. G Ital Nefrol. 2005. PMID: 16419001 Review. Italian.
Cited by
-
B Cell in Autoimmune Diseases.Scientifica (Cairo). 2012;2012:215308. doi: 10.6064/2012/215308. Scientifica (Cairo). 2012. PMID: 23807906 Free PMC article.
-
Have we overestimated the benefit of human(ized) antibodies?MAbs. 2010 Nov-Dec;2(6):682-94. doi: 10.4161/mabs.2.6.13601. Epub 2010 Nov 1. MAbs. 2010. PMID: 20935511 Free PMC article. Review.
-
Antigen-specific immunotherapies in rheumatic diseases.Nat Rev Rheumatol. 2017 Sep;13(9):525-537. doi: 10.1038/nrrheum.2017.107. Epub 2017 Jul 13. Nat Rev Rheumatol. 2017. PMID: 28701761 Review.
-
A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1).Clin Exp Immunol. 2005 May;140(2):230-40. doi: 10.1111/j.1365-2249.2005.02764.x. Clin Exp Immunol. 2005. PMID: 15807846 Free PMC article.
-
The inhibition of anti-DNA binding to DNA by nucleic acid binding polymers.PLoS One. 2012;7(7):e40862. doi: 10.1371/journal.pone.0040862. Epub 2012 Jul 11. PLoS One. 2012. PMID: 22808279 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
