Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury
- PMID: 15302676
- PMCID: PMC1575259
- DOI: 10.1038/sj.bjp.0705862
Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury
Abstract
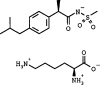
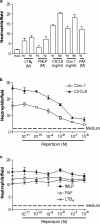
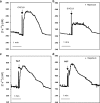
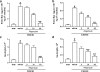
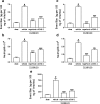
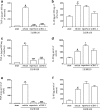
1. Neutrophils are thought to play a major role in the mediation of reperfusion injury. CXC chemokines are known inducers of neutrophil recruitment. Here, we assessed the effects of Repertaxin, a novel low molecular weight inhibitor of human CXCL8 receptor activation, on the local, remote and systemic injuries following intestinal ischaemia and reperfusion (I/R) in the rat. 2. Pre-incubation of rat neutrophils with Repertaxin (10(-11)-10(-6) m) inhibited the chemotaxis of neutrophils induced by human CXCL8 or rat CINC-1, but not that induced by fMLP, PAF or LTB(4), in a concentration-dependent manner. Repertaxin also prevented CXCL8-induced calcium influx but not CXCL8 binding to purified rat neutrophils. 2. In a model of mild I/R injury (30 min of ischaemia and 30 min of reperfusion), Repertaxin dose-dependently (3-30 mg kg(-1)) inhibited the increase in vascular permeability and neutrophil influx. Maximal inhibition occurred at 30 mg kg(-1). 4. Following severe I/R injury (120 min of ischaemia and 120 min of reperfusion), Repertaxin (30 mg kg(-1)) markedly prevented neutrophil influx, the increase in vascular permeability both in the intestine and the lungs. Moreover, there was prevention of haemorrhage in the intestine of reperfused animals. 5. Repertaxin effectively suppressed the increase in tissue (intestine and lungs) and serum concentrations of TNF-alpha and the reperfusion-associated lethality. 6. For comparison, we also evaluated the effects of an anti-CINC-1 antibody in the model of severe I/R injury. Overall, the antibody effectively prevented tissue injury, systemic inflammation and lethality. However, the effects of the antibody were in general of lower magnitude than those of Repertaxin. 7. In conclusion, CINC-1 and possibly other CXC chemokines, acting on CXCR2, have an important role during I/R injury. Thus, drugs, such as Repertaxin, developed to block the function of the CXCR2 receptor may be effective at preventing reperfusion injury in relevant clinical situations.
Figures

Similar articles
-
APT070 (Mirococept), a membrane-localised complement inhibitor, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury.Br J Pharmacol. 2005 Aug;145(8):1027-34. doi: 10.1038/sj.bjp.0706286. Br J Pharmacol. 2005. PMID: 15951831 Free PMC article.
-
Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion.Kidney Int. 2005 May;67(5):1753-61. doi: 10.1111/j.1523-1755.2005.00272.x. Kidney Int. 2005. PMID: 15840022
-
Effects of neutrophil elastase inhibitor (ONO-5046) on lung injury after intestinal ischemia-reperfusion.J Appl Physiol (1985). 2001 Oct;91(4):1800-7. doi: 10.1152/jappl.2001.91.4.1800. J Appl Physiol (1985). 2001. PMID: 11568165
-
[The role of rat cytokine-induced neutrophil chemoattractants (CINCs) in inflammation].Yakugaku Zasshi. 2002 Apr;122(4):263-8. doi: 10.1248/yakushi.122.263. Yakugaku Zasshi. 2002. PMID: 11968838 Review. Japanese.
-
Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation.Pulm Pharmacol Ther. 2015 Oct;34:37-45. doi: 10.1016/j.pupt.2015.08.002. Epub 2015 Aug 10. Pulm Pharmacol Ther. 2015. PMID: 26271598 Review.
Cited by
-
CXCR2: a target for pancreatic cancer treatment?Expert Opin Ther Targets. 2013 Jun;17(6):667-80. doi: 10.1517/14728222.2013.772137. Epub 2013 Feb 21. Expert Opin Ther Targets. 2013. PMID: 23425074 Free PMC article. Review.
-
Blockade of CXCR1/2 chemokine receptors protects against brain damage in ischemic stroke in mice.Clinics (Sao Paulo). 2013;68(3):391-4. doi: 10.6061/clinics/2013(03)oa17. Clinics (Sao Paulo). 2013. PMID: 23644861 Free PMC article.
-
Role of CINC-1 and CXCR2 receptors on LPS-induced fever in rats.Pflugers Arch. 2019 Feb;471(2):301-311. doi: 10.1007/s00424-018-2222-0. Epub 2018 Oct 22. Pflugers Arch. 2019. PMID: 30349936
-
Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury.J Surg Res. 2007 May 15;139(2):269-73. doi: 10.1016/j.jss.2006.10.047. Epub 2007 Feb 7. J Surg Res. 2007. PMID: 17291530 Free PMC article.
-
Vascular cell-adhesion molecule 1 (VCAM-1) regulates JunB-mediated IL-8/CXCL1 expression and pathological neovascularization.Commun Biol. 2023 May 13;6(1):516. doi: 10.1038/s42003-023-04905-z. Commun Biol. 2023. PMID: 37179352 Free PMC article.
References
-
- BAGGIOLINI M., LOETSCHER P., MOSER B. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 1995;17:103–108. - PubMed
-
- BAXTER G.F. The neutrophil as a mediator of myocardial ischemia–reperfusion injury: time to move on. Basic Res. Cardiol. 2002;97:268–275. - PubMed
-
- BERTINI R., ALLEGRETTI M., BIZZARRI C., MORICONI A., LOCATI M., ZAMPELLA G., CERVELLERA M.N., DI CIOCCIO V., CESTA M.C., GALLIERA E., MARTINEZ F.O., DI BITONDO R., TROIANI G., SABBATINI V., D'ANNIBALLE G., ANACARDIO R., CUTRIN J.C., CAVALIERI B., MAINIERO F., STRIPPOLI R., VILLA P., DI GIROLAMO M., MARTIN F., GENTILE M., SANTONI A., CORDA D., GHEZZI P., POLI G., MANTOVANI A., COLOTTA F.A new class of non-competitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury Proc. Nat. Acad. Sci. U.S.A. 2004(in press) - PMC - PubMed
-
- BIGNOLD L.P., FERRANTE A. Mechanism of separation of polymorphonuclear leukocytes from whole blood by the one-step Hypaque–Ficoll method. J. Immunol. Methods. 1987;96:29–33. - PubMed
-
- BIZZARRI C., BERTINI R., BOSSÙ P., SOZZANI S., MANTOVANI A., VAN DAMME J., TAGLIABUE A., BORASCHI D. Single-cell analysis of macrophage chemotactic protein-1-regulated cytosolic Ca2+ increase in human adherent monocytes. Blood. 1995;86:2388–2394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

