Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function
- PMID: 15289494
- PMCID: PMC2172255
- DOI: 10.1083/jcb.200312168
Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function
Abstract
Transformation of fibroblasts by oncogenic Src causes disruption of actin stress fibers and formation of invasive adhesions called podosomes. Because the small GTPase Rho stimulates stress fiber formation, Rho inactivation by Src has been thought to be necessary for stress fiber disruption. However, we show here that Rho[GTP] levels do not decrease after transformation by activated Src. Inactivation of Rho in Src-transformed fibroblasts by dominant negative RhoA or the Rho-specific inhibitor C3 exoenzyme disrupted podosome structure as judged by localization of podosome components F-actin, cortactin, and Fish. Inhibition of Rho strongly inhibited Src-induced proteolytic degradation of the extracellular matrix. Furthermore, development of an in situ Rho[GTP] affinity assay allowed us to detect endogenous Rho[GTP] at podosomes, where it colocalized with F-actin, cortactin, and Fish. Therefore, Rho is not globally inactivated in Src-transformed fibroblasts, but is necessary for the assembly and function of structures implicated in tumor cell invasion.
Figures

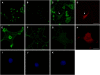
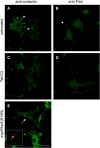
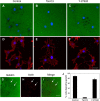
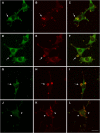
Similar articles
-
ERK5 promotes Src-induced podosome formation by limiting Rho activation.J Cell Biol. 2008 Jun 30;181(7):1195-210. doi: 10.1083/jcb.200801078. Epub 2008 Jun 23. J Cell Biol. 2008. PMID: 18573916 Free PMC article.
-
Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts.Cancer Res. 2002 Feb 1;62(3):669-74. Cancer Res. 2002. PMID: 11830518
-
The guanine nucleotide exchange factor Arhgef5 plays crucial roles in Src-induced podosome formation.J Cell Sci. 2011 May 15;124(Pt 10):1726-38. doi: 10.1242/jcs.080291. Epub 2011 Apr 26. J Cell Sci. 2011. PMID: 21525037
-
Rho GTPases in osteoclasts: orchestrators of podosome arrangement.Eur J Cell Biol. 2008 Sep;87(8-9):469-77. doi: 10.1016/j.ejcb.2008.03.002. Epub 2008 Apr 23. Eur J Cell Biol. 2008. PMID: 18436334 Review.
-
Podosome and sealing zone: specificity of the osteoclast model.Eur J Cell Biol. 2006 Apr;85(3-4):195-202. doi: 10.1016/j.ejcb.2005.09.008. Epub 2005 Oct 24. Eur J Cell Biol. 2006. PMID: 16546562 Review.
Cited by
-
Ubiquitous membrane-bound DNase activity in podosomes and invadopodia.J Cell Biol. 2021 Jul 5;220(7):e202008079. doi: 10.1083/jcb.202008079. J Cell Biol. 2021. PMID: 33904858 Free PMC article.
-
How to make a static cytokinetic furrow out of traveling excitable waves.Small GTPases. 2016 Apr 2;7(2):65-70. doi: 10.1080/21541248.2016.1168505. Epub 2016 Apr 12. Small GTPases. 2016. PMID: 27070950 Free PMC article. Review.
-
Identification and characterization of Dlc1 isoforms in the mouse and study of the biological function of a single gene trapped isoform.BMC Biol. 2010 Mar 3;8:17. doi: 10.1186/1741-7007-8-17. BMC Biol. 2010. PMID: 20199662 Free PMC article.
-
Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14353-8. doi: 10.1073/pnas.0807537105. Epub 2008 Sep 17. Proc Natl Acad Sci U S A. 2008. PMID: 18799748 Free PMC article.
-
Invadopodia and basement membrane invasion in vivo.Cell Adh Migr. 2014;8(3):246-55. doi: 10.4161/cam.28406. Cell Adh Migr. 2014. PMID: 24717190 Free PMC article. Review.
References
-
- Abram, C.L., D.F. Seals, I. Pass, D. Salinsky, L. Maurer, T.M. Roth, and S.A. Courtneidge. 2003. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 278:16844–16851. - PubMed
-
- Burns, S., A.J. Thrasher, M.P. Blundell, L. Machesky, and G.E. Jones. 2001. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 98:1142–1149. - PubMed
-
- Chellaiah, M.A., N. Soga, S. Swanson, S. McAllister, U. Alvarez, D. Wang, S.F. Dowdy, and K.A. Hruska. 2000. b. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275:11993–12002. - PubMed
-
- Chen, W.T. 1989. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 251:167–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

