The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology
- PMID: 15289291
- PMCID: PMC1575337
- DOI: 10.1038/sj.bjp.0705930
The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology
Abstract
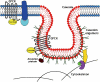
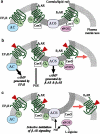
The many components of G-protein-coupled receptor (GPCR) signal transduction provide cells with numerous combinations with which to customize their responses to hormones, neurotransmitters, and pharmacologic agonists. GPCRs function as guanine nucleotide exchange factors for heterotrimeric (alpha, beta, gamma) G proteins, thereby promoting exchange of GTP for GDP and, in turn, the activation of 'downstream' signaling components. Recent data indicate that individual cells express mRNA for perhaps over 100 different GPCRs (out of a total of nearly a thousand GPCR genes), several different combinations of G-protein subunits, multiple regulators of G-protein signaling proteins (which function as GTPase activating proteins), and various isoforms of downstream effector molecules. The differential expression of such protein combinations allows for modulation of signals that are customized for a specific cell type, perhaps at different states of maturation or differentiation. In addition, in the linear arrangement of molecular interactions involved in a given GPCR-G-protein-effector pathway, one needs to consider the localization of receptors and post-receptor components in subcellular compartments, microdomains, and molecular complexes, and to understand the movement of proteins between these compartments. Co-localization of signaling components, many of which are expressed at low overall concentrations, allows cells to tailor their responses by arranging, or spatially organizing in unique and kinetically favorable ways, the molecules involved in GPCR signal transduction. This review focuses on the role of lipid rafts and a subpopulation of such rafts, caveolae, as a key spatial compartment enriched in components of GPCR signal transduction. Recent data suggest cell-specific patterns for expression of those components in lipid rafts and caveolae. Such domains likely define functionally important, cell-specific regions of signaling by GPCRs and drugs active at those GPCRs.
Figures

Similar articles
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. Update in: Commun Chem. 2024 Nov 19;7(1):271. doi: 10.1038/s42004-024-01352-7. PMID: 38260445 Free PMC article. Updated. Preprint.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
A Small Number of Surgeons Perform the Large Majority of Uncommon Nerve Decompression Procedures.Clin Orthop Relat Res. 2024 Dec 1;482(12):2182-2190. doi: 10.1097/CORR.0000000000003162. Epub 2024 Jun 21. Clin Orthop Relat Res. 2024. PMID: 38905446
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Caveolin-1 regulates osteoclastogenesis and bone metabolism in a sex-dependent manner.J Biol Chem. 2015 Mar 6;290(10):6522-30. doi: 10.1074/jbc.M114.598581. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561739 Free PMC article.
-
Quantitative phosphoproteomic analysis reveals unique cAMP signaling pools emanating from AC2 and AC6 in human airway smooth muscle cells.Front Physiol. 2023 Feb 28;14:1149063. doi: 10.3389/fphys.2023.1149063. eCollection 2023. Front Physiol. 2023. PMID: 36926196 Free PMC article.
-
Peptide G-Protein-Coupled Receptors and ErbB Receptor Tyrosine Kinases in Cancer.Biology (Basel). 2023 Jul 4;12(7):957. doi: 10.3390/biology12070957. Biology (Basel). 2023. PMID: 37508387 Free PMC article. Review.
-
Functional characterization of AC5 gain-of-function variants: Impact on the molecular basis of ADCY5-related dyskinesia.Biochem Pharmacol. 2019 May;163:169-177. doi: 10.1016/j.bcp.2019.02.005. Epub 2019 Feb 14. Biochem Pharmacol. 2019. PMID: 30772269 Free PMC article.
-
Ligand-induced tyrosine phosphorylation of cysteinyl leukotriene receptor 1 triggers internalization and signaling in intestinal epithelial cells.PLoS One. 2010 Dec 28;5(12):e14439. doi: 10.1371/journal.pone.0014439. PLoS One. 2010. PMID: 21203429 Free PMC article.
References
-
- ALOUSI A.A., JASPER J.R., INSEL P.A., MOTULSKY H.J. Stoichiometry of receptor-Gs-adenylate cyclase interactions. FASEB J. 1991;5:2300–2303. - PubMed
-
- ANDERSON R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- ANDERSON R.G., JACOBSON K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. - PubMed
-
- AWASTHI-KALIA M., SCHNETKAMP P.P., DEANS J.P. Differential effects of filipin and methyl-beta-cyclodextrin on B cell receptor signaling. Biochem. Biophys. Res. Commun. 2001;287:77–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

