Tapasin enhances MHC class I peptide presentation according to peptide half-life
- PMID: 15286279
- PMCID: PMC511045
- DOI: 10.1073/pnas.0306294101
Tapasin enhances MHC class I peptide presentation according to peptide half-life
Abstract
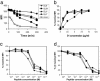
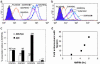
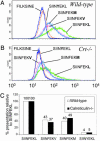
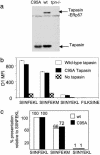
Understanding how peptides are selected for presentation by MHC class I is crucial to vaccination strategies based on cytotoxic T lymphocyte priming. We have studied this selection of the MHC class I peptide repertoire in terms of the presentation of a series of individual peptides with a wide range of binding to MHC class I. This series was expressed as minigenes, and the presentation of each peptide variant was determined with the same MHC class I peptide-specific antibody. In wild-type cells, the hierarchy of presentation followed peptide half-life. This hierarchy broke down in cells lacking tapasin but not in cells lacking calreticulin or in cells lacking transporter associated with antigen processing-associated ERp57. We demonstrate a key role for tapasin in shaping the MHC class I peptide repertoire, as enhancement of presentation in the presence of tapasin correlated with peptide half-life.
Figures
Similar articles
-
The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex.Immunol Rev. 2005 Oct;207:89-99. doi: 10.1111/j.0105-2896.2005.00311.x. Immunol Rev. 2005. PMID: 16181329 Review.
-
Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer.Nat Immunol. 2007 Aug;8(8):873-81. doi: 10.1038/ni1485. Epub 2007 Jul 1. Nat Immunol. 2007. PMID: 17603487
-
Impaired immune responses and altered peptide repertoire in tapasin-deficient mice.Nat Immunol. 2000 Sep;1(3):234-8. doi: 10.1038/79775. Nat Immunol. 2000. PMID: 10973281
-
Molecular architecture of the MHC I peptide-loading complex: one tapasin molecule is essential and sufficient for antigen processing.FASEB J. 2012 Dec;26(12):5071-80. doi: 10.1096/fj.12-217489. Epub 2012 Aug 24. FASEB J. 2012. PMID: 22923333
-
Interaction of ERp57 and tapasin in the generation of MHC class I-peptide complexes.Curr Opin Immunol. 2007 Feb;19(1):99-105. doi: 10.1016/j.coi.2006.11.013. Epub 2006 Dec 5. Curr Opin Immunol. 2007. PMID: 17150345 Review.
Cited by
-
Synergism of tapasin and human leukocyte antigens in resolving hepatitis C virus infection.Hepatology. 2013 Sep;58(3):881-9. doi: 10.1002/hep.26415. Epub 2013 Jul 29. Hepatology. 2013. PMID: 23532923 Free PMC article.
-
Engineering superior DNA vaccines: MHC class I single chain trimers bypass antigen processing and enhance the immune response to low affinity antigens.Vaccine. 2010 Feb 23;28(8):1911-8. doi: 10.1016/j.vaccine.2009.10.096. Vaccine. 2010. PMID: 20188246 Free PMC article.
-
Pseudomonas aeruginosa Cif protein enhances the ubiquitination and proteasomal degradation of the transporter associated with antigen processing (TAP) and reduces major histocompatibility complex (MHC) class I antigen presentation.J Biol Chem. 2014 Jan 3;289(1):152-62. doi: 10.1074/jbc.M113.459271. Epub 2013 Nov 18. J Biol Chem. 2014. PMID: 24247241 Free PMC article.
-
Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment.J Immunol. 2011 Nov 1;187(9):4403-9. doi: 10.4049/jimmunol.1101531. J Immunol. 2011. PMID: 22013206 Free PMC article. Review.
-
Comparative analysis of the impact of a free cysteine in tapasin on the maturation and surface expression of murine MHC class I allotypes.Int J Immunogenet. 2009 Jun;36(3):183-7. doi: 10.1111/j.1744-313X.2009.00840.x. Int J Immunogenet. 2009. PMID: 19490214 Free PMC article.
References
-
- Shastri, N., Schwab, S. & Serwold, T. (2002) Annu. Rev. Immunol. 20, 463–493. - PubMed
-
- Engelhard, V. H., Brickner, A. G. & Zarling, A. L. (2002) Mol. Immunol. 39, 127–137. - PubMed
-
- Madden, D. R. (1995) Annu. Rev. Immunol. 13, 587–622. - PubMed
-
- Williams, A., Peh, C. A. & Elliott, T. (2002) Tissue Antigens 59, 3–17. - PubMed
-
- Ortmann, B., Copeman, J., Lehner, P. J., Sadasivan, B., Herberg, J. A., Grandea, A. G., Riddell, S. R., Tampe, R., Spies, T., Trowsdale, J. & Cresswell, P. (1997) Science 277, 1306–1309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

