Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease
- PMID: 15285796
- PMCID: PMC483059
- DOI: 10.1186/1742-2094-1-6
Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease
Abstract
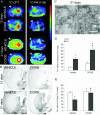
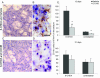
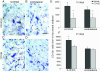
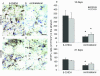
Several lines of evidence point to a significant role of neuroinflammation in Parkinson's disease (PD) and other neurodegenerative disorders. In the present study we examined the protective effect of celecoxib, a selective inhibitor of the inducible form of cyclooxygenase (COX-2), on dopamine (DA) cell loss in a rat model of PD. We used the intrastriatal administration of 6-hydroxydopamine (6-OHDA) that induces a retrograde neuronal damage and death, which progresses over weeks. Animals were randomized to receive celecoxib (20 mg/kg/day) or vehicle starting 1 hour before the intrastriatal administration of 6-OHDA. Evaluation was performed in vivo using micro PET and selective radiotracers for DA terminals and microglia. Post mortem analysis included stereological quantification of tyrosine hydroxylase, astrocytes and microglia. 12 days after the 6-OHDA lesion there were no differences in DA cell or fiber loss between groups, although the microglial cell density and activation was markedly reduced in animals receiving celecoxib (p < 0.01). COX-2 inhibition did not reduce the typical astroglial response in the striatum at any stage. Between 12 and 21 days, there was a significant progression of DA cell loss in the vehicle group (from 40 to 65%) that was prevented by celecoxib. Therefore, inhibition of COX-2 by celecoxib appears to be able, either directly or through inhibition of microglia activation to prevent or slow down DA cell degeneration.
Figures
Similar articles
-
Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease.J Neurosci. 2006 Sep 13;26(37):9365-75. doi: 10.1523/JNEUROSCI.1504-06.2006. J Neurosci. 2006. PMID: 16971520 Free PMC article.
-
Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson's disease.Neuroscience. 2008 Feb 19;151(4):1142-53. doi: 10.1016/j.neuroscience.2007.11.036. Epub 2007 Dec 4. Neuroscience. 2008. PMID: 18201835
-
Intrastriatal administration of erythropoietin protects dopaminergic neurons and improves neurobehavioral outcome in a rat model of Parkinson's disease.Neuroscience. 2007 May 25;146(3):1245-58. doi: 10.1016/j.neuroscience.2007.02.004. Epub 2007 Mar 23. Neuroscience. 2007. PMID: 17363174
-
Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor.Exp Neurol. 1998 Dec;154(2):261-75. doi: 10.1006/exnr.1998.6887. Exp Neurol. 1998. PMID: 9878166
-
Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson's disease.Neurobiol Dis. 2006 May;22(2):421-34. doi: 10.1016/j.nbd.2005.12.008. Epub 2006 Feb 9. Neurobiol Dis. 2006. PMID: 16480889
Cited by
-
Neuroinflammation in Parkinson's Disease and its Treatment Opportunities.Balkan Med J. 2022 Sep 9;39(5):318-333. doi: 10.4274/balkanmedj.galenos.2022.2022-7-100. Epub 2022 Aug 29. Balkan Med J. 2022. PMID: 36036436 Free PMC article.
-
Celecoxib attenuates systemic lipopolysaccharide-induced brain inflammation and white matter injury in the neonatal rats.Neuroscience. 2013 Jun 14;240:27-38. doi: 10.1016/j.neuroscience.2013.02.041. Epub 2013 Feb 26. Neuroscience. 2013. PMID: 23485816 Free PMC article.
-
Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets.Signal Transduct Target Ther. 2023 Sep 22;8(1):359. doi: 10.1038/s41392-023-01588-0. Signal Transduct Target Ther. 2023. PMID: 37735487 Free PMC article. Review.
-
The degenerating substantia nigra as a susceptible region for gene transfer-mediated inflammation.Parkinsons Dis. 2011;2011:931572. doi: 10.4061/2011/931572. Epub 2011 May 29. Parkinsons Dis. 2011. PMID: 21687774 Free PMC article.
-
Pretreatment with crocin along with treadmill exercise ameliorates motor and memory deficits in hemiparkinsonian rats by anti-inflammatory and antioxidant mechanisms.Metab Brain Dis. 2019 Apr;34(2):459-468. doi: 10.1007/s11011-018-0379-z. Epub 2019 Jan 16. Metab Brain Dis. 2019. PMID: 30652256
References
-
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. - PubMed
-
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

