Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification
- PMID: 15282552
- PMCID: PMC514519
- DOI: 10.1038/sj.emboj.7600346
Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification
Abstract
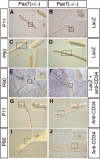
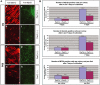
The paired-box transcription factor Pax7 has been claimed to specify the muscle stem cell lineage since inactivation of Pax7 led to a failure to detect muscle satellite cells. Here we show that muscles of juvenile Pax7(-/-) mice at P11 contain a reduced but substantial number of satellite cells. Neither juvenile nor adult Pax7(-/-) mice displayed a significant reduction in the number and size of myotubes, indicating that the remaining number of satellite cells sufficed to allow normal postnatal muscle growth. The number of satellite cells in Pax7 mutant mice declined strongly during postnatal development, although single satellite cells were readily identified in adult Pax7 mutant mice. Muscle regeneration was impaired in adult Pax7 mutant mice. Our results clearly indicate an essential function of Pax7 for renewal and maintenance of muscle stem cells and exclude an exclusive role of Pax7 in satellite cell specification.
Figures





Similar articles
-
Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal.Dev Dyn. 2004 Nov;231(3):489-502. doi: 10.1002/dvdy.20151. Dev Dyn. 2004. PMID: 15390217
-
Myogenic specification of side population cells in skeletal muscle.J Cell Biol. 2002 Oct 14;159(1):123-34. doi: 10.1083/jcb.200202092. Epub 2002 Oct 14. J Cell Biol. 2002. PMID: 12379804 Free PMC article.
-
Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development.Nat Cell Biol. 2010 Mar;12(3):257-66. doi: 10.1038/ncb2025. Epub 2010 Jan 31. Nat Cell Biol. 2010. PMID: 20118923
-
The molecular regulation of muscle stem cell function.Cold Spring Harb Symp Quant Biol. 2008;73:323-31. doi: 10.1101/sqb.2008.73.064. Epub 2009 Mar 27. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19329572 Review.
-
Defining the transcriptional signature of skeletal muscle stem cells.J Anim Sci. 2008 Apr;86(14 Suppl):E207-16. doi: 10.2527/jas.2007-0473. Epub 2007 Sep 18. J Anim Sci. 2008. PMID: 17878281 Free PMC article. Review.
Cited by
-
Barx2 and Pax7 Regulate Axin2 Expression in Myoblasts by Interaction with β-Catenin and Chromatin Remodelling.Stem Cells. 2016 Aug;34(8):2169-82. doi: 10.1002/stem.2396. Epub 2016 Jun 6. Stem Cells. 2016. PMID: 27144473 Free PMC article.
-
Acetylation of PAX7 controls muscle stem cell self-renewal and differentiation potential in mice.Nat Commun. 2021 May 31;12(1):3253. doi: 10.1038/s41467-021-23577-z. Nat Commun. 2021. PMID: 34059674 Free PMC article.
-
The skeletal muscle satellite cell: still young and fascinating at 50.J Histochem Cytochem. 2011 Dec;59(12):1041-59. doi: 10.1369/0022155411426780. J Histochem Cytochem. 2011. PMID: 22147605 Free PMC article.
-
Muscle development, regeneration and laminopathies: how lamins or lamina-associated proteins can contribute to muscle development, regeneration and disease.Cell Mol Life Sci. 2013 Aug;70(15):2713-41. doi: 10.1007/s00018-012-1190-3. Epub 2012 Nov 10. Cell Mol Life Sci. 2013. PMID: 23138638 Free PMC article. Review.
-
Embracing change: striated-for-smooth muscle replacement in esophagus development.Skelet Muscle. 2016 Aug 8;6:27. doi: 10.1186/s13395-016-0099-1. eCollection 2016. Skelet Muscle. 2016. PMID: 27504178 Free PMC article. Review.
References
-
- Bancroft JD, Stevens A (1990) Theory and Practice of Histological Techniques. Edinburgh, New York: Churchill Livingstone
-
- Barr FG (1999) The role of chimeric paired box transcription factors in the pathogenesis of pediatric rhabdomysarcoma. Cancer Res 59: 1711s–1715s - PubMed
-
- Bischoff R (1994) The satellite cell and muscle regeneration. In Myogenesis, Engel AG, Franszini-Armstrong C (eds) pp 97–118. New York: McGraw-Hill
-
- Braun T, Rudnicki MA, Arnold HH, Jaenisch R (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71: 369–382 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

