Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein
- PMID: 15282282
- PMCID: PMC6729726
- DOI: 10.1523/JNEUROSCI.1826-04.2004
Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein
Abstract
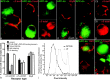
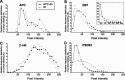
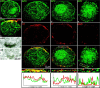
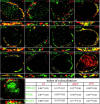
Normal cognitive and autonomic functions require nicotinic synaptic signaling. Despite the physiological importance of these synapses, little is known about molecular mechanisms that direct their assembly during development. We show here that the tumor-suppressor protein adenomatous polyposis coli (APC) functions in localizing alpha3-nicotinic acetylcholine receptors (nAChRs) to neuronal postsynaptic sites. Our quantitative confocal microscopy studies indicate that APC is selectively enriched at cholinergic synapses; APC surface clusters are juxtaposed to synaptic vesicle clusters and colocalize with alpha3-nAChRs but not with the neighboring synaptic glycine receptors or perisynaptic alpha7-nAChRs on chick ciliary ganglion (CG) neurons. We identify PSD (postsynaptic density)-93, beta-catenin, and microtubule end binding protein EB1 as APC binding partners. PSD-93 and beta-catenin are also enriched at alpha3-nAChR postsynaptic sites. EB1 shows close proximity to and partial overlap with alpha3-nAChR and APC surface clusters. We tested the role of APC in neuronal nicotinic synapse assembly by using retroviral-mediated in vivo overexpression of an APC dominant-negative (APC-dn) peptide to block the interaction of endogenous APC with both EB1 and PSD-93 during synapse formation in CG neurons. The overexpressed APC-dn led to dramatic decreases in alpha3-nAChR surface levels and clusters. Effects were specific to alpha3-nAChR postsynaptic sites; synaptic glycine receptor and perisynaptic alpha7-nAChR clusters were not altered. In addition, APC-dn also reduced surface membrane-associated clusters of PSD-93 and EB1. The results show that APC plays a key role in organizing excitatory cholinergic postsynaptic specializations in CG neurons. We identify APC as the first nonreceptor protein to function in localizing nAChRs to neuronal synapses in vivo.
Figures

Similar articles
-
Adenomatous polyposis coli plays a key role, in vivo, in coordinating assembly of the neuronal nicotinic postsynaptic complex.Mol Cell Neurosci. 2008 Jun;38(2):138-52. doi: 10.1016/j.mcn.2008.02.006. Epub 2008 Mar 4. Mol Cell Neurosci. 2008. PMID: 18407517 Free PMC article.
-
The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo.J Neurosci. 2010 Aug 18;30(33):11073-85. doi: 10.1523/JNEUROSCI.0983-10.2010. J Neurosci. 2010. PMID: 20720115 Free PMC article.
-
Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function.J Neurosci. 2004 May 5;24(18):4340-50. doi: 10.1523/JNEUROSCI.0055-04.2004. J Neurosci. 2004. PMID: 15128848 Free PMC article.
-
Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses.Mol Neurobiol. 2002 Feb;25(1):79-112. doi: 10.1385/MN:25:1:079. Mol Neurobiol. 2002. PMID: 11890459 Review.
-
Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo.J Physiol. 2000 May 15;525 Pt 1(Pt 1):21-9. doi: 10.1111/j.1469-7793.2000.00021.x. J Physiol. 2000. PMID: 10811721 Free PMC article. Review.
Cited by
-
WNTs tune up the neuromuscular junction.Nat Rev Neurosci. 2009 Sep;10(9):627-34. doi: 10.1038/nrn2681. Nat Rev Neurosci. 2009. PMID: 19693027 Free PMC article. Review.
-
Lateral mobility of nicotinic acetylcholine receptors on neurons is determined by receptor composition, local domain, and cell type.J Neurosci. 2010 Jun 30;30(26):8841-51. doi: 10.1523/JNEUROSCI.6236-09.2010. J Neurosci. 2010. PMID: 20592206 Free PMC article.
-
Expression and Manipulation of the APC-β-Catenin Pathway During Peripheral Neuron Regeneration.Sci Rep. 2018 Sep 4;8(1):13197. doi: 10.1038/s41598-018-31167-1. Sci Rep. 2018. PMID: 30181617 Free PMC article.
-
EphB receptors co-distribute with a nicotinic receptor subtype and regulate nicotinic downstream signaling in neurons.Mol Cell Neurosci. 2008 Jun;38(2):236-44. doi: 10.1016/j.mcn.2008.02.013. Epub 2008 Mar 18. Mol Cell Neurosci. 2008. PMID: 18403216 Free PMC article.
-
Wnt signaling in neuromuscular junction development.Cold Spring Harb Perspect Biol. 2012 Jun;4(6):a008045. doi: 10.1101/cshperspect.a008045. Cold Spring Harb Perspect Biol. 2012. PMID: 22510459 Free PMC article. Review.
References
-
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE (1999) The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol 9: 425-428. - PubMed
-
- Bienz M (2002) The subcellular destinations of APC proteins. Nat Rev Mol Cell Biol 3: 328-338. - PubMed
-
- Brakeman JS, Gu SH, Wang XB, Dolin G, Baraban JM (1999) Neuronal localization of the Adenomatous polyposis coli tumor suppressor protein. Neuroscience 91: 661-672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

