Evidence that stable retroviral transduction and cell survival following DNA integration depend on components of the nonhomologous end joining repair pathway
- PMID: 15280466
- PMCID: PMC479090
- DOI: 10.1128/JVI.78.16.8573-8581.2004
Evidence that stable retroviral transduction and cell survival following DNA integration depend on components of the nonhomologous end joining repair pathway
Abstract
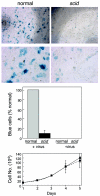
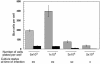
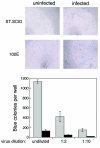
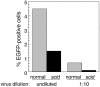
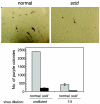
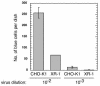
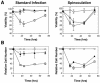
We have previously reported several lines of evidence that support a role for cellular DNA repair systems in completion of the retroviral DNA integration process. Failure to repair an intermediate in the process of integrating viral DNA into host DNA appears to trigger growth arrest or death of a large percentage of infected cells. Cellular proteins involved in the nonhomologous end joining (NHEJ) pathway (DNA-PK(CS)) and the damage-signaling kinases (ATM and ATR) have been implicated in this process. However, some studies have suggested that NHEJ proteins may not be required for the completion of lentiviral DNA integration. Here we provide additional evidence that NHEJ proteins are required for stable transduction by human immunodeficiency type 1 (HIV-1)-based vectors. Our analyses with two different reporters show that the number of stably transduced DNA-PK(CS)-deficient scid fibroblasts was reduced by 80 to 90% compared to the number of control cells. Furthermore, transduction efficiency can be restored to wild-type levels in scid cells that are complemented with a functional DNA-PK(CS) gene. The efficiency of stable transduction by an HIV-1-based vector is also reduced upon infection of Xrcc4 and ligase IV-deficient cells, implying a role for these components of the NHEJ repair pathway. Finally, we show that cells deficient in ligase IV are killed by infection with an integrase-competent but not an integrase-deficient HIV-1 vector. Results presented in this study lend further support to a general role for the NHEJ DNA repair pathway in completion of the retroviral DNA integration process.
Figures
Similar articles
-
Retroviral DNA integration and the DNA damage response.Cell Death Differ. 2005 Aug;12 Suppl 1:971-8. doi: 10.1038/sj.cdd.4401573. Cell Death Differ. 2005. PMID: 15761474 Review.
-
Wortmannin potentiates integrase-mediated killing of lymphocytes and reduces the efficiency of stable transduction by retroviruses.Mol Cell Biol. 2001 Feb;21(4):1164-72. doi: 10.1128/MCB.21.4.1164-1172.2001. Mol Cell Biol. 2001. PMID: 11158303 Free PMC article.
-
A role for DNA-PK in retroviral DNA integration.Science. 1999 Apr 23;284(5414):644-7. doi: 10.1126/science.284.5414.644. Science. 1999. PMID: 10213687
-
DNA-Dependent protein kinase is not required for efficient lentivirus integration.J Virol. 2000 Dec;74(23):11278-85. doi: 10.1128/jvi.74.23.11278-11285.2000. J Virol. 2000. PMID: 11070027 Free PMC article.
-
Mechanisms of DNA double strand break repair and chromosome aberration formation.Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review.
Cited by
-
Host Proteins Ku and HMGA1 As Participants of HIV-1 Transcription.Acta Naturae. 2016 Jan-Mar;8(1):34-47. Acta Naturae. 2016. PMID: 27099783 Free PMC article.
-
Positive selection of yeast nonhomologous end-joining genes and a retrotransposon conflict hypothesis.Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17614-9. doi: 10.1073/pnas.0605468103. Epub 2006 Nov 13. Proc Natl Acad Sci U S A. 2006. PMID: 17101967 Free PMC article.
-
NHEJ pathway is involved in post-integrational DNA repair due to Ku70 binding to HIV-1 integrase.Retrovirology. 2019 Nov 6;16(1):30. doi: 10.1186/s12977-019-0492-z. Retrovirology. 2019. PMID: 31690330 Free PMC article.
-
An end-joining repair mechanism in Escherichia coli.Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):2141-6. doi: 10.1073/pnas.0906355107. Epub 2010 Jan 19. Proc Natl Acad Sci U S A. 2010. PMID: 20133858 Free PMC article.
-
Rapid evolution of BRCA1 and BRCA2 in humans and other primates.BMC Evol Biol. 2014 Jul 11;14:155. doi: 10.1186/1471-2148-14-155. BMC Evol Biol. 2014. PMID: 25011685 Free PMC article.
References
-
- Chen, W. Y., X. Wu, D. N. Levasseur, H. Liu, L. Lai, J. C. Kappes, and T. M. Townes. 2000. Lentiviral vector transduction of hematopoietic stem cells that mediate long-term reconstitution of lethally irradiated mice. Stem Cells 18:352-359. - PubMed
-
- Coffin, J. M., S. H. Hughes, and H. E. Varmus. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. - PubMed
-
- Craig, N. L. 1996. V(D)J recombination and transposition: closer than expected. Science 271:1512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

