Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation
- PMID: 15272039
- PMCID: PMC1665187
- DOI: 10.1113/jphysiol.2004.067637
Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation
Abstract
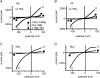
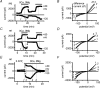
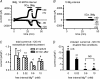
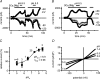
Cardiac tissue expresses several TRP proteins as well as a Mg2+ -inhibited, non-selective cation current (IMIC) that bears many characteristics of TRP channel currents. We used the whole-cell voltage clamp technique in pig and rat ventricular myocytes to characterize the permeation, blockage properties and regulation of the cardiac IMIC channels in order to compare them with TRP channels, in particular with Mg2+ -sensitive TRPM6 and TRPM7. We show that removing extracellular divalent cations unmasks large inward and outward monovalent currents, which can be inhibited by intracellular Mg2+. Inward currents are suppressed upon replacing extracellular Na+ by NMDG+. Divalent cations block monovalent IMIC and, at 10-20 mm, carry measurable currents. Their efficacy sequence in decreasing outward IMIC (Ni2+ = Mg2+ > Ca2+ > Ba2+) and in inducing inward IMIC (Ni2+ >> Mg2+ = Ca2+ approximately Ba2+), and their permeabilities calculated from reversal potentials are similar to those of TRPM6 and TRPM7 channels. The trivalent cations Gd3+ and Dy3+ also block IMIC in a voltage-dependent manner (delta = 0.4-0.5). In addition they inhibit the inward current carried by divalent cations. IMIC is regulated by pH. Decreasing or increasing extracellular pH decreased and increased IMIC, respectively (pH0.5 = 6.9, nH = 0.98). Qualitatively similar results were obtained on IMIC in rat basophilic leukaemia cells. These effects in cardiac myocytes were absent in the presence of high intracellular buffering by 40 mm Hepes. Our results suggest that IMIC in cardiac cells is due to TRPM channels, most probably to TRPM6 or TRPM7 channels or to their heteromultimeres.
Figures


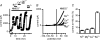

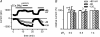

Similar articles
-
Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle.J Physiol. 1997 Jul 15;502 ( Pt 2)(Pt 2):235-47. doi: 10.1111/j.1469-7793.1997.235bk.x. J Physiol. 1997. PMID: 9263906 Free PMC article.
-
Modulation of Human Cardiac TRPM7 Current by Extracellular Acidic pH Depends upon Extracellular Concentrations of Divalent Cations.PLoS One. 2017 Jan 27;12(1):e0170923. doi: 10.1371/journal.pone.0170923. eCollection 2017. PLoS One. 2017. PMID: 28129376 Free PMC article.
-
Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7.J Biol Chem. 2007 Aug 31;282(35):25817-30. doi: 10.1074/jbc.M608972200. Epub 2007 Jun 28. J Biol Chem. 2007. PMID: 17599911 Free PMC article.
-
The Mg2+ and Mg(2+)-nucleotide-regulated channel-kinase TRPM7.Handb Exp Pharmacol. 2007;(179):313-28. doi: 10.1007/978-3-540-34891-7_19. Handb Exp Pharmacol. 2007. PMID: 17217066 Free PMC article. Review.
-
Molecular components of vertebrate Mg2+-homeostasis regulation.Magnes Res. 2007 Mar;20(1):6-18. Magnes Res. 2007. PMID: 17536484 Review.
Cited by
-
TRPM7.Handb Exp Pharmacol. 2014;222:521-46. doi: 10.1007/978-3-642-54215-2_21. Handb Exp Pharmacol. 2014. PMID: 24756720 Free PMC article. Review.
-
Characterization of Mg²⁺-regulated TRPM7-like current in human atrial myocytes.J Biomed Sci. 2012 Aug 14;19(1):75. doi: 10.1186/1423-0127-19-75. J Biomed Sci. 2012. PMID: 22891975 Free PMC article.
-
Control of cardiac contraction by sodium: Promises, reckonings, and new beginnings.Cell Calcium. 2020 Jan;85:102129. doi: 10.1016/j.ceca.2019.102129. Epub 2019 Nov 22. Cell Calcium. 2020. PMID: 31835176 Free PMC article. Review.
-
TRPM7, Magnesium, and Signaling.Int J Mol Sci. 2019 Apr 16;20(8):1877. doi: 10.3390/ijms20081877. Int J Mol Sci. 2019. PMID: 30995736 Free PMC article. Review.
-
Evidence for the expression of TRPM6 and TRPM7 in cardiomyocytes from all four chamber walls of the human heart.Sci Rep. 2021 Jul 29;11(1):15445. doi: 10.1038/s41598-021-94856-4. Sci Rep. 2021. PMID: 34326388 Free PMC article.
References
-
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. - PubMed
-
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–173. - PubMed
-
- Bakowski D, Parekh AB. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current ICRAC in rat basophilic leukemia cells. Pflugers Arch. 2002;443:892–902. - PubMed
-
- Bosteels S, Matejovic P, Flameng W, Mubagwa K. Sodium influx via a non-selective pathway activated by the removal of extracellular divalent cations: possible role in the calcium paradox. Cardiovasc Res. 1999;43:417–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

