An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus
- PMID: 15247913
- PMCID: PMC7095806
- DOI: 10.1038/nm1080
An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus
Abstract
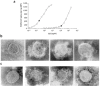
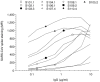
Passive serotherapy can confer immediate protection against microbial infection, but methods to rapidly generate human neutralizing monoclonal antibodies are not yet available. We have developed an improved method for Epstein-Barr virus transformation of human B cells. We used this method to analyze the memory repertoire of a patient who recovered from severe acute respiratory syndrome coronavirus (SARS-CoV) infection and to isolate monoclonal antibodies specific for different viral proteins, including 35 antibodies with in vitro neutralizing activity ranging from 10(-8)M to 10(-11)M. One such antibody confers protection in vivo in a mouse model of SARS-CoV infection. These results show that it is possible to interrogate the memory repertoire of immune donors to rapidly and efficiently isolate neutralizing antibodies that have been selected in the course of natural infection.
Conflict of interest statement
The Institute for Research in Biomedicine has a patent application that covers the method described in this article.
Figures
Similar articles
-
The spike protein of severe acute respiratory syndrome (SARS) is cleaved in virus infected Vero-E6 cells.Cell Res. 2004 Oct;14(5):400-6. doi: 10.1038/sj.cr.7290240. Cell Res. 2004. PMID: 15450134 Free PMC article.
-
Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients.Clin Immunol. 2006 Aug;120(2):171-8. doi: 10.1016/j.clim.2006.05.002. Epub 2006 Jun 16. Clin Immunol. 2006. PMID: 16781892 Free PMC article.
-
Understanding and making use of human memory B cells.Immunol Rev. 2006 Jun;211(1):303-9. doi: 10.1111/j.0105-2896.2006.00403.x. Immunol Rev. 2006. PMID: 16824137 Free PMC article. Review.
-
Human monoclonal antibodies by immortalization of memory B cells.Curr Opin Biotechnol. 2007 Dec;18(6):523-8. doi: 10.1016/j.copbio.2007.10.011. Epub 2007 Dec 11. Curr Opin Biotechnol. 2007. PMID: 18063358 Free PMC article. Review.
-
Human B cell memory.Curr Opin Immunol. 2009 Jun;21(3):298-304. doi: 10.1016/j.coi.2009.05.019. Epub 2009 Jun 6. Curr Opin Immunol. 2009. PMID: 19497721 Free PMC article. Review.
Cited by
-
Development of a new genotype-phenotype linked antibody screening system.Elife. 2024 Nov 19;13:RP95346. doi: 10.7554/eLife.95346. Elife. 2024. PMID: 39558690 Free PMC article.
-
Defective germinal center selection results in persistence of self-reactive B cells from the primary to the secondary repertoire in Primary Antiphospholipid Syndrome.Nat Commun. 2024 Nov 15;15(1):9921. doi: 10.1038/s41467-024-54228-8. Nat Commun. 2024. PMID: 39548093 Free PMC article.
-
Monoclonal antibodies: From magic bullet to precision weapon.Mol Biomed. 2024 Oct 11;5(1):47. doi: 10.1186/s43556-024-00210-1. Mol Biomed. 2024. PMID: 39390211 Free PMC article. Review.
-
Broadly potent spike-specific human monoclonal antibodies inhibit SARS-CoV-2 Omicron sub-lineages.Commun Biol. 2024 Oct 2;7(1):1239. doi: 10.1038/s42003-024-06951-7. Commun Biol. 2024. PMID: 39354108 Free PMC article.
-
Mutational Patterns Observed in SARS-CoV-2 Genomes Sampled From Successive Epochs Delimited by Major Public Health Events in Ontario, Canada: Genomic Surveillance Study.JMIR Bioinform Biotechnol. 2022 Dec 22;3(1):e42243. doi: 10.2196/42243. JMIR Bioinform Biotechnol. 2022. PMID: 38935965 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

