Myofibroblast development is characterized by specific cell-cell adherens junctions
- PMID: 15240821
- PMCID: PMC515361
- DOI: 10.1091/mbc.e04-05-0386
Myofibroblast development is characterized by specific cell-cell adherens junctions
Abstract
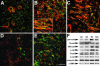
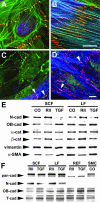
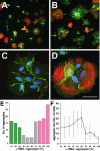
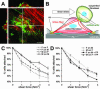
Myofibroblasts of wound granulation tissue, in contrast to dermal fibroblasts, join stress fibers at sites of cadherin-type intercellular adherens junctions (AJs). However, the function of myofibroblast AJs, their molecular composition, and the mechanisms of their formation are largely unknown. We demonstrate that fibroblasts change cadherin expression from N-cadherin in early wounds to OB-cadherin in contractile wounds, populated with alpha-smooth muscle actin (alpha-SMA)-positive myofibroblasts. A similar shift occurs during myofibroblast differentiation in culture and seems to be responsible for the homotypic segregation of alpha-SMA-positive and -negative fibroblasts in suspension. AJs of plated myofibroblasts are reinforced by alpha-SMA-mediated contractile activity, resulting in high mechanical resistance as demonstrated by subjecting cell pairs to hydrodynamic forces in a flow chamber. A peptide that inhibits alpha-SMA-mediated contractile force causes the reorganization of large stripe-like AJs to belt-like contacts as shown for enhanced green fluorescent protein-alpha-catenin-transfected cells and is associated with a reduced mechanical resistance. Anti-OB-cadherin but not anti-N-cadherin peptides reduce the contraction of myofibroblast-populated collagen gels, suggesting that AJs are instrumental for myofibroblast contractile activity.
Figures



Similar articles
-
The myofibroblast, cadherin, alpha smooth muscle actin and the collagen effect.Cell Biochem Funct. 2006 Jan-Feb;24(1):63-70. doi: 10.1002/cbf.1188. Cell Biochem Funct. 2006. PMID: 15584087
-
The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo.J Cell Biol. 2002 May 13;157(4):657-63. doi: 10.1083/jcb.200201049. Epub 2002 May 6. J Cell Biol. 2002. PMID: 11994316 Free PMC article.
-
Ligand-activated PPARδ upregulates α-smooth muscle actin expression in human dermal fibroblasts: A potential role for PPARδ in wound healing.J Dermatol Sci. 2015 Dec;80(3):186-95. doi: 10.1016/j.jdermsci.2015.10.005. Epub 2015 Oct 9. J Dermatol Sci. 2015. PMID: 26481780
-
Myofibroblasts in palatal wound healing: prospects for the reduction of wound contraction after cleft palate repair.J Dent Res. 2005 Oct;84(10):871-80. doi: 10.1177/154405910508401002. J Dent Res. 2005. PMID: 16183784 Review.
-
Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling.Thromb Haemost. 2003 Dec;90(6):993-1002. doi: 10.1160/TH03-05-0328. Thromb Haemost. 2003. PMID: 14652629 Review.
Cited by
-
Acute contact with profibrotic macrophages mechanically activates fibroblasts via αvβ3 integrin-mediated engagement of Piezo1.Sci Adv. 2024 Oct 25;10(43):eadp4726. doi: 10.1126/sciadv.adp4726. Epub 2024 Oct 23. Sci Adv. 2024. PMID: 39441936 Free PMC article.
-
Molecular networks underlying myofibroblast fate and fibrosis.J Mol Cell Cardiol. 2016 Aug;97:153-61. doi: 10.1016/j.yjmcc.2016.05.002. Epub 2016 May 7. J Mol Cell Cardiol. 2016. PMID: 27167848 Free PMC article. Review.
-
Mitochondrial fission and bioenergetics mediate human lung fibroblast durotaxis.JCI Insight. 2023 Jan 10;8(1):e157348. doi: 10.1172/jci.insight.157348. JCI Insight. 2023. PMID: 36422990 Free PMC article.
-
Pericryptal myofibroblast growth in rat descending colon induced by low-sodium diets is mediated by aldosterone and not by angiotensin II.J Membr Biol. 2005 Jul;206(1):53-9. doi: 10.1007/s00232-005-0773-4. J Membr Biol. 2005. PMID: 16440181
-
Characterization of cell subpopulations expressing progenitor cell markers in porcine cardiac valves.PLoS One. 2013 Jul 23;8(7):e69667. doi: 10.1371/journal.pone.0069667. Print 2013. PLoS One. 2013. PMID: 23936071 Free PMC article.
References
-
- Adams, C.L., and Nelson, W.J. (1998). Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10, 572–577. - PubMed
-
- Angst, B.D., Marcozzi, C., and Magee, A.I. (2001). The cadherin superfamily: diversity in form and function. J. Cell Sci. 114, 629–641. - PubMed
-
- Bershadsky, A.D., Balaban, N.Q., and Geiger, B. (2003). Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19, 677–695. - PubMed
-
- Bochaton-Piallat, M.-L., Ropraz, P., Gabbiani, F., and Gabbiani, G. (1996). Phenotypic heterogeneity of rat arterial smooth muscle cell clones - implications for the development of experimental intimal thickening. Arterioscler. Thromb. Vasc. Biol. 16, 815–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

