Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor
- PMID: 15240571
- PMCID: PMC2172142
- DOI: 10.1083/jcb.200310098
Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor
Erratum in
- J Cell Biol. 2004 Nov 8;167(3):following 562
Abstract
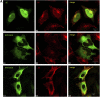
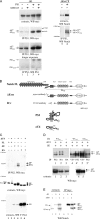
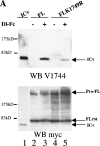
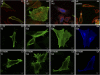
Activation of mammalian Notch receptor by its ligands induces TNFalpha-converting enzyme-dependent ectodomain shedding, followed by intramembrane proteolysis due to presenilin (PS)-dependent gamma-secretase activity. Here, we demonstrate that a new modification, a monoubiquitination, as well as clathrin-dependent endocytosis, is required for gamma-secretase processing of a constitutively active Notch derivative, DeltaE, which mimics the TNFalpha-converting enzyme-processing product. PS interacts with this modified form of DeltaE, DeltaEu. We identified the lysine residue targeted by the monoubiquitination event and confirmed its importance for activation of Notch receptor by its ligand, Delta-like 1. We propose a new model where monoubiquitination and endocytosis of Notch are a prerequisite for its PS-dependent cleavage, and discuss its relevance for other gamma-secretase substrates.
Figures





Similar articles
-
The Notch ligands, Jagged and Delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments.J Biol Chem. 2003 Sep 5;278(36):34427-37. doi: 10.1074/jbc.M302659200. Epub 2003 Jun 25. J Biol Chem. 2003. PMID: 12826675
-
Conserved "PAL" sequence in presenilins is essential for gamma-secretase activity, but not required for formation or stabilization of gamma-secretase complexes.Neurobiol Dis. 2004 Apr;15(3):654-66. doi: 10.1016/j.nbd.2003.12.008. Neurobiol Dis. 2004. PMID: 15056474
-
Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s).Biochemistry. 2003 Jan 14;42(1):137-44. doi: 10.1021/bi026888g. Biochemistry. 2003. PMID: 12515548
-
Presenilin function and gamma-secretase activity.J Neurochem. 2005 May;93(4):769-92. doi: 10.1111/j.1471-4159.2005.03099.x. J Neurochem. 2005. PMID: 15857382 Review.
-
Intramembrane proteolysis by presenilin and presenilin-like proteases.J Cell Sci. 2003 Jul 15;116(Pt 14):2839-44. doi: 10.1242/jcs.00651. J Cell Sci. 2003. PMID: 12808018 Review.
Cited by
-
The role of endocytosis in activating and regulating signal transduction.Cell Mol Life Sci. 2012 Jun;69(11):1755-71. doi: 10.1007/s00018-011-0877-1. Epub 2011 Nov 24. Cell Mol Life Sci. 2012. PMID: 22113372 Free PMC article. Review.
-
GIT1 protects against breast cancer growth through negative regulation of Notch.Nat Commun. 2022 Mar 22;13(1):1537. doi: 10.1038/s41467-022-28631-y. Nat Commun. 2022. PMID: 35318302 Free PMC article.
-
Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1.Mol Cell Biol. 2008 Jan;28(1):165-76. doi: 10.1128/MCB.00863-07. Epub 2007 Oct 29. Mol Cell Biol. 2008. PMID: 17967888 Free PMC article.
-
Notch signaling as a master regulator of adult neurogenesis.Front Neurosci. 2023 Jun 29;17:1179011. doi: 10.3389/fnins.2023.1179011. eCollection 2023. Front Neurosci. 2023. PMID: 37457009 Free PMC article. Review.
-
A Notch updated.J Cell Biol. 2009 Mar 9;184(5):621-9. doi: 10.1083/jcb.200811141. Epub 2009 Mar 2. J Cell Biol. 2009. PMID: 19255248 Free PMC article. Review.
References
-
- Annaert, W.G., L. Levesque, K. Craessaerts, I. Dierinck, G. Snellings, D. Westaway, P.S. George-Hyslop, B. Cordell, P. Fraser, and B. De Strooper. 1999. Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J. Cell Biol. 147:277–294. - PMC - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303–1311. - PubMed
-
- Bland, C.E., P. Kimberly, and M.D. Rand. 2003. Notch-induced proteolysis and nuclear localization of the Delta ligand. J. Biol. Chem. 278:13607–13610. - PubMed
-
- Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J.R. Doedens, A. Cumano, P. Roux, R.A. Black, and A. Israël. 2000. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol. Cell. 5:207–216. - PubMed
-
- Buttner, C., S. Sadtler, A. Leyendecker, B. Laube, N. Griffon, H. Betz, and G. Schmalzing. 2001. Ubiquitination precedes internalization and proteolytic cleavage of plasma membrane-bound glycine receptors. J. Biol. Chem. 276:42978–42985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

