Ca2+ activates human homologous recombination protein Rad51 by modulating its ATPase activity
- PMID: 15226506
- PMCID: PMC454202
- DOI: 10.1073/pnas.0402105101
Ca2+ activates human homologous recombination protein Rad51 by modulating its ATPase activity
Abstract
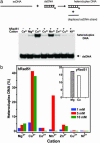
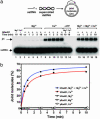
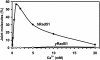
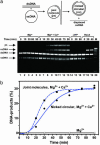
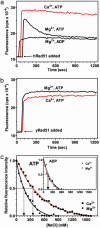
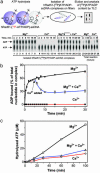
Human Rad51 (hRad51) protein plays a key role in homologous recombination and DNA repair. hRad51 protein forms a helical filament on single-stranded DNA (ssDNA), which performs the basic steps of homologous recombination: a search for homologous double-stranded DNA (dsDNA) and DNA strand exchange. hRad51 protein possesses DNA-dependent ATPase activity; however, the role of this activity has not been understood. Our current results show that Ca(2+) greatly stimulates DNA strand exchange activity of hRad51 protein. We found that Ca(2+) exerts its stimulatory effect by modulating the ATPase activity of hRad51 protein. Our data demonstrate that, in the presence of Mg(2+), the hRad51-ATP-ssDNA filament is quickly converted to an inactive hRad51-ADP-ssDNA form, due to relatively rapid ATP hydrolysis and slow dissociation of ADP. Ca(2+) maintains the active hRad51-ATP-ssDNA filament by reducing the ATP hydrolysis rate. These findings demonstrate a crucial role of the ATPase activity in regulation of DNA strand exchange activity of hRad51 protein. This mechanism of Rad51 protein regulation by modulating its ATPase activity is evolutionarily recent; we found no such mechanism for yeast Rad51 (yRad51) protein.
Figures
Similar articles
-
Biochemical characterization of the human RAD51 protein. I. ATP hydrolysis.J Biol Chem. 2002 Apr 26;277(17):14417-25. doi: 10.1074/jbc.M109915200. Epub 2002 Feb 11. J Biol Chem. 2002. PMID: 11839739
-
Defining the salt effect on human RAD51 activities.DNA Repair (Amst). 2006 Jun 10;5(6):718-30. doi: 10.1016/j.dnarep.2006.03.006. Epub 2006 Apr 27. DNA Repair (Amst). 2006. PMID: 16644292
-
Roles of ATP binding and ATP hydrolysis in human Rad51 recombinase function.DNA Repair (Amst). 2006 Mar 7;5(3):381-91. doi: 10.1016/j.dnarep.2005.11.005. Epub 2006 Jan 4. DNA Repair (Amst). 2006. PMID: 16388992
-
Human Rad54 protein stimulates DNA strand exchange activity of hRad51 protein in the presence of Ca2+.J Biol Chem. 2004 Dec 10;279(50):52042-51. doi: 10.1074/jbc.M410244200. Epub 2004 Oct 4. J Biol Chem. 2004. PMID: 15466868
-
Regulation of DNA strand exchange in homologous recombination.DNA Repair (Amst). 2010 Dec 10;9(12):1264-72. doi: 10.1016/j.dnarep.2010.09.014. DNA Repair (Amst). 2010. PMID: 20971042 Review.
Cited by
-
Saccharomyces cerevisiae Dmc1 and Rad51 proteins preferentially function with Tid1 and Rad54 proteins, respectively, to promote DNA strand invasion during genetic recombination.J Biol Chem. 2012 Aug 17;287(34):28727-37. doi: 10.1074/jbc.M112.373290. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22761450 Free PMC article.
-
Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress.Mol Cell. 2012 Aug 10;47(3):396-409. doi: 10.1016/j.molcel.2012.05.024. Epub 2012 Jun 14. Mol Cell. 2012. PMID: 22704558 Free PMC article.
-
A mutant form of Dmc1 that bypasses the requirement for accessory protein Mei5-Sae3 reveals independent activities of Mei5-Sae3 and Rad51 in Dmc1 filament stability.PLoS Genet. 2019 Dec 2;15(12):e1008217. doi: 10.1371/journal.pgen.1008217. eCollection 2019 Dec. PLoS Genet. 2019. PMID: 31790385 Free PMC article.
-
Stimulation of fission yeast and mouse Hop2-Mnd1 of the Dmc1 and Rad51 recombinases.Nucleic Acids Res. 2007;35(8):2719-33. doi: 10.1093/nar/gkm174. Epub 2007 Apr 10. Nucleic Acids Res. 2007. PMID: 17426123 Free PMC article.
-
MDM2 antagonists promote CRISPR/Cas9-mediated precise genome editing in sheep primary cells.Mol Ther Nucleic Acids. 2023 Jan 2;31:309-323. doi: 10.1016/j.omtn.2022.12.020. eCollection 2023 Mar 14. Mol Ther Nucleic Acids. 2023. PMID: 36726409 Free PMC article.
References
-
- West, S. C. (2003) Nat. Rev. Mol. Cell. Biol. 4, 435-445. - PubMed
-
- Sung, P., Krejci, L., Van Komen, S. & Sehorn, M. G. (2003) J. Biol. Chem. 278, 42729-42732. - PubMed
-
- Kowalczykowski, S. C. (2002) Nat. Struct. Biol. 9, 897-899. - PubMed
-
- Hassold, T. & Sherman, S. (2000) Clin. Genet. 57, 95-100. - PubMed
-
- Masson, J. Y. & West, S. C. (2001) Trends Biochem. Sci. 26, 131-136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

