A novel severe acute respiratory syndrome coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis
- PMID: 15194747
- PMCID: PMC421683
- DOI: 10.1128/JVI.78.13.6723-6734.2004
A novel severe acute respiratory syndrome coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis
Abstract
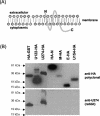
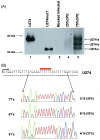
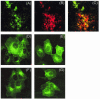
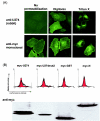
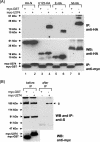
The severe acute respiratory syndrome coronavirus (SARS-CoV) genome contains open reading frames (ORFs) that encode for several genes that are homologous to proteins found in all known coronaviruses. These are the replicase gene 1a/1b and the four structural proteins, nucleocapsid (N), spike (S), membrane (M), and envelope (E), and these proteins are expected to be essential for the replication of the virus. In addition, this genome also contains nine other potential ORFs varying in length from 39 to 274 amino acids. The largest among these is the first ORF of the second longest subgenomic RNA, and this protein (termed U274 in the present study) consists of 274 amino acids and contains three putative transmembrane domains. Using antibody specific for the C terminus of U274, we show U274 to be expressed in SARS-CoV-infected Vero E6 cells and, in addition to the full-length protein, two other processed forms were also detected. By indirect immunofluorescence, U274 was localized to the perinuclear region, as well as to the plasma membrane, in both transfected and infected cells. Using an N terminus myc-tagged U274, the topology of U274 and its expression on the cell surface were confirmed. Deletion of a cytoplasmic domain of U274, which contains Yxxphi and diacidic motifs, abolished its transport to the cell surface. In addition, U274 expressed on the cell surface can internalize antibodies from the culture medium into the cells. Coimmunoprecipitation experiments also showed that U274 could interact specifically with the M, E, and S structural proteins, as well as with U122, another protein that is unique to SARS-CoV.
Figures

Similar articles
-
Characterization of a unique group-specific protein (U122) of the severe acute respiratory syndrome coronavirus.J Virol. 2004 Jul;78(14):7311-8. doi: 10.1128/JVI.78.14.7311-7318.2004. J Virol. 2004. PMID: 15220404 Free PMC article.
-
Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway.J Virol. 2004 Dec;78(24):14043-7. doi: 10.1128/JVI.78.24.14043-14047.2004. J Virol. 2004. PMID: 15564512 Free PMC article.
-
Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins.J Virol. 2004 Sep;78(18):9977-86. doi: 10.1128/JVI.78.18.9977-9986.2004. J Virol. 2004. PMID: 15331731 Free PMC article.
-
Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus.Antiviral Res. 2006 Nov;72(2):78-88. doi: 10.1016/j.antiviral.2006.05.010. Epub 2006 Jun 6. Antiviral Res. 2006. PMID: 16820226 Free PMC article. Review.
-
Characterization of viral proteins encoded by the SARS-coronavirus genome.Antiviral Res. 2005 Feb;65(2):69-78. doi: 10.1016/j.antiviral.2004.10.001. Antiviral Res. 2005. PMID: 15708633 Free PMC article. Review.
Cited by
-
SARS-CoV-2 ORF3a Protein as a Therapeutic Target against COVID-19 and Long-Term Post-Infection Effects.Pathogens. 2024 Jan 14;13(1):75. doi: 10.3390/pathogens13010075. Pathogens. 2024. PMID: 38251382 Free PMC article. Review.
-
Endoplasmic reticulum-associated SARS-CoV-2 ORF3a elicits heightened cytopathic effects despite robust ER-associated degradation.mBio. 2024 Jan 16;15(1):e0303023. doi: 10.1128/mbio.03030-23. Epub 2023 Dec 11. mBio. 2024. PMID: 38078754 Free PMC article.
-
The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity.iScience. 2023 Sep 28;26(11):108080. doi: 10.1016/j.isci.2023.108080. eCollection 2023 Nov 17. iScience. 2023. PMID: 37860693 Free PMC article.
-
Some aspects of the life of SARS-CoV-2 ORF3a protein in mammalian cells.Heliyon. 2023 Aug 1;9(8):e18754. doi: 10.1016/j.heliyon.2023.e18754. eCollection 2023 Aug. Heliyon. 2023. PMID: 37609425 Free PMC article.
-
Coronavirus accessory protein ORF3 biology and its contribution to viral behavior and pathogenesis.iScience. 2023 Apr 21;26(4):106280. doi: 10.1016/j.isci.2023.106280. Epub 2023 Feb 28. iScience. 2023. PMID: 36945252 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

