Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice
- PMID: 15194645
- PMCID: PMC1774108
- DOI: 10.1136/gut.2003.027136
Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice
Abstract
Background: Leptin regulates appetite through the long isoform of its receptor in the hypothalamus. Although leptin regulates immune responses, it is still unknown whether a direct effect of leptin on lymphocytes is required.
Aims: To clarify whether expression of leptin receptors on T lymphocytes modulates intestinal inflammation in mice.
Methods: The model of colitis induced by transfer of CD4(+)CD45RB(high) (RB(high)) cells into scid mice was used. Wild-type (WT) or leptin receptor deficient (db/db) RB(high) cells were transferred into scid mice and development of colitis evaluated.
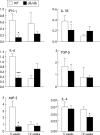
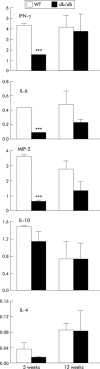
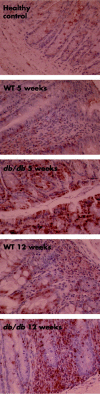
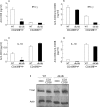
Results: Leptin receptors were expressed on both RB(high) and RB(low) cells. Intestinal lymphocytes of mice with colitis expressed high leptin levels compared with healthy controls whereas the opposite was true for serum leptin levels. Transfer of RB(high) cells from db/db mice induced delayed disease compared with transfer of WT cells. A high rate of apoptosis in lamina propria lymphocytes and reduced cytokine production were observed early on in scid mice receiving db/db RB(high) cells. These effects were not due to the high levels of glucocorticoids present in db/db mice as administration of corticosterone to WT mice failed to reproduce this phenomenon. High expression of peroxisome proliferator activated receptor gamma was observed in the colon of recipients of db/db compared with WT cells. Freshly isolated db/db RB(high) cells produced low levels of interferon gamma. Despite delayed onset of colitis, as disease progressed differences between mice receiving WT or db/db cells were no longer apparent.
Conclusions: These results suggest that leptin affects the immune response, partly by acting on the long isoform of its receptor expressed on T lymphocytes.
Figures
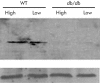
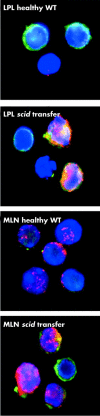
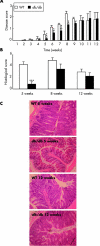
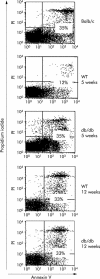
Comment in
-
Leptin in intestinal inflammation: good and bad gut feelings.Gut. 2004 Jul;53(7):921-2. doi: 10.1136/gut.2003.037283. Gut. 2004. PMID: 15194634 Free PMC article.
Similar articles
-
Defining the role of T cell-derived leptin in the modulation of hepatic or intestinal inflammation in mice.Clin Exp Immunol. 2005 Oct;142(1):31-8. doi: 10.1111/j.1365-2249.2005.02898.x. Clin Exp Immunol. 2005. PMID: 16178853 Free PMC article.
-
Development of antigen induced colitis in SCID mice reconstituted with spleen derived memory type CD4(+) CD45RB(+) T cells.Gut. 2002 Mar;50(3):299-306. doi: 10.1136/gut.50.3.299. Gut. 2002. PMID: 11839705 Free PMC article.
-
Reciprocal regulation of the survival and apoptosis of Th17 and Th1 cells in the colon.Inflamm Bowel Dis. 2012 Feb;18(2):333-43. doi: 10.1002/ibd.21772. Epub 2011 May 25. Inflamm Bowel Dis. 2012. PMID: 21618360
-
Cytokine regulation of experimental intestinal inflammation in genetically engineered and T-lymphocyte reconstituted rodents.Aliment Pharmacol Ther. 1996;10 Suppl 2:36-42; discussion 43-4. doi: 10.1046/j.1365-2036.1996.22164018.x. Aliment Pharmacol Ther. 1996. PMID: 8899099 Review.
-
The weight of leptin in immunity.Nat Rev Immunol. 2004 May;4(5):371-9. doi: 10.1038/nri1350. Nat Rev Immunol. 2004. PMID: 15122202 Review.
Cited by
-
Leptin and Inflammation.Curr Immunol Rev. 2008 May 1;4(2):70-79. doi: 10.2174/157339508784325046. Curr Immunol Rev. 2008. PMID: 20198122 Free PMC article.
-
Role of Leptin in the Digestive System.Front Pharmacol. 2021 Apr 12;12:660040. doi: 10.3389/fphar.2021.660040. eCollection 2021. Front Pharmacol. 2021. PMID: 33935782 Free PMC article. Review.
-
Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells.Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5150-5. doi: 10.1073/pnas.0408995102. Epub 2005 Mar 23. Proc Natl Acad Sci U S A. 2005. PMID: 15788534 Free PMC article.
-
The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease.Cells. 2019 Jun 21;8(6):628. doi: 10.3390/cells8060628. Cells. 2019. PMID: 31234447 Free PMC article. Review.
-
Adipokines and the role of visceral adipose tissue in inflammatory bowel disease.Ann Gastroenterol. 2016 Oct-Dec;29(4):424-438. doi: 10.20524/aog.2016.0077. Epub 2016 Sep 6. Ann Gastroenterol. 2016. PMID: 27708507 Free PMC article. Review.
References
-
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. - PubMed
-
- Fantuzzi G , Faggioni R. Leptin in the regulation of immunity, inflammation and hematopoiesis. J Leukoc Biol 2000;68:437–46. - PubMed
-
- Busso N , So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 2002;168:875–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
