TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH
- PMID: 15190109
- PMCID: PMC6729305
- DOI: 10.1523/JNEUROSCI.0890-04.2004
TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH
Abstract
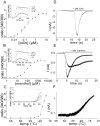
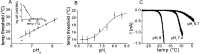
TRPM8 is a nonselective cation channel activated by cold and the cooling compounds menthol and icilin (Peier et al., 2002). Here, we have used electrophysiology and the calcium-sensitive dye Fura-2 to study the effect of pH and interactions between temperature, pH, and the two chemical agonists menthol and icilin on TRPM8 expressed in Chinese hamster ovary cells. Menthol, icilin, and cold all evoked stimulus-dependent [Ca2+]i responses in standard physiological solutions of pH 7.3. Increasing the extracellular [H+] from pH 7.3 to approximately pH 6 abolished responses to icilin and cold stimulation but did not affect responses to menthol. Icilin concentration-response curves were significantly shifted to the right when pH was lowered from 7.3 to 6.9, whereas those with menthol were unaltered in solutions of pH 6.1. When cells were exposed to solutions in the range of pH 8.1-6.5, the temperature threshold for activation was elevated at higher pH and depressed at lower pH. Superfusing cells with a low subactivating concentration of icilin or menthol elevated the threshold for cold activation at pH 7.4, but cooling failed to evoke [Ca2+]i responses at pH 6 in the presence of either agonist. In voltage-clamp experiments in which the intracellular pH was buffered to different levels, acidification reduced the current amplitude of icilin responses and shifted the threshold for cold activation to lower values with half-maximal inhibition at pH 7.2 and pH 7.6. The results demonstrate that the activation of TRPM8 by icilin and cold, but not menthol, is modulated by intracellular pH in the physiological range. Furthermore, our data suggest that activation by icilin and cold involve a different mechanism to activation by menthol.
Figures

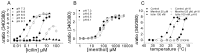

Similar articles
-
The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity.Mol Pain. 2010 Jan 21;6:4. doi: 10.1186/1744-8069-6-4. Mol Pain. 2010. PMID: 20092626 Free PMC article.
-
The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel.Neuron. 2004 Sep 16;43(6):859-69. doi: 10.1016/j.neuron.2004.08.038. Neuron. 2004. PMID: 15363396
-
Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives.J Biol Chem. 2009 Feb 13;284(7):4102-11. doi: 10.1074/jbc.M806651200. Epub 2008 Dec 18. J Biol Chem. 2009. PMID: 19095656
-
Modulation of thermoreceptor TRPM8 by cooling compounds.ACS Chem Neurosci. 2012 Apr 18;3(4):248-67. doi: 10.1021/cn300006u. Epub 2012 Feb 13. ACS Chem Neurosci. 2012. PMID: 22860192 Free PMC article. Review.
-
Mutations of TRPM8 channels: Unraveling the molecular basis of activation by cold and ligands.Med Res Rev. 2022 Nov;42(6):2168-2203. doi: 10.1002/med.21920. Epub 2022 Aug 17. Med Res Rev. 2022. PMID: 35976012 Free PMC article. Review.
Cited by
-
Supercooling agent icilin blocks a warmth-sensing ion channel TRPV3.ScientificWorldJournal. 2012;2012:982725. doi: 10.1100/2012/982725. Epub 2012 Apr 1. ScientificWorldJournal. 2012. PMID: 22548000 Free PMC article.
-
Progress in the Structural Basis of thermoTRP Channel Polymodal Gating.Int J Mol Sci. 2023 Jan 1;24(1):743. doi: 10.3390/ijms24010743. Int J Mol Sci. 2023. PMID: 36614186 Free PMC article. Review.
-
Competitive Interactions between PIRT, the Cold Sensing Ion Channel TRPM8, and PIP2 Suggest a Mechanism for Regulation.Sci Rep. 2019 Oct 1;9(1):14128. doi: 10.1038/s41598-019-49912-5. Sci Rep. 2019. PMID: 31575973 Free PMC article.
-
The Concise Guide to PHARMACOLOGY 2013/14: ion channels.Br J Pharmacol. 2013 Dec;170(8):1607-51. doi: 10.1111/bph.12447. Br J Pharmacol. 2013. PMID: 24528239 Free PMC article.
-
Expression of temperature-sensitive ion channel TRPM8 in sperm cells correlates with vertebrate evolution.PeerJ. 2015 Oct 13;3:e1310. doi: 10.7717/peerj.1310. eCollection 2015. PeerJ. 2015. PMID: 26500819 Free PMC article.
References
-
- Galla JH (2000) Metabolic alkalosis. J Am Soc Nephrol 11: 369-375. - PubMed
-
- Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A (2000) Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem 275: 32552-32558. - PubMed
-
- Heppner TJ, Bonev AD, Santana LF, Nelson MT (2002) Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurized cerebral arteries. Am J Physiol Heart Circ Physiol 283: H2169-H2176. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
