Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis
- PMID: 15181211
- PMCID: PMC514130
- DOI: 10.1104/pp.104.038711
Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis
Abstract
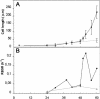
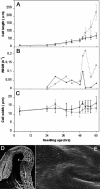
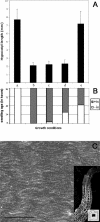
A central problem in plant biology is how cell expansion is coordinated with wall synthesis. We have studied growth and wall deposition in epidermal cells of dark-grown Arabidopsis hypocotyls. Cells elongated in a biphasic pattern, slowly first and rapidly thereafter. The growth acceleration was initiated at the hypocotyl base and propagated acropetally. Using transmission and scanning electron microscopy, we analyzed walls in slowly and rapidly growing cells in 4-d-old dark-grown seedlings. We observed thick walls in slowly growing cells and thin walls in rapidly growing cells, which indicates that the rate of cell wall synthesis was not coupled to the cell elongation rate. The thick walls showed a polylamellated architecture, whereas polysaccharides in thin walls were axially oriented. Interestingly, innermost cellulose microfibrils were transversely oriented in both slowly and rapidly growing cells. This suggested that transversely deposited microfibrils reoriented in deeper layers of the expanding wall. No growth acceleration, only slow growth, was observed in the cellulose synthase mutant cesA6(prc1-1) or in seedlings, which had been treated with the cellulose synthesis inhibitor isoxaben. In these seedlings, innermost microfibrils were transversely oriented and not randomized as has been reported for other cellulose-deficient mutants or following treatment with dichlorobenzonitrile. Interestingly, isoxaben treatment after the initiation of the growth acceleration in the hypocotyl did not affect subsequent cell elongation. Together, these results show that rapid cell elongation, which involves extensive remodeling of the cell wall polymer network, depends on normal cellulose deposition during the slow growth phase.
Figures


Similar articles
-
Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein.Plant Cell. 2002 Sep;14(9):2145-60. doi: 10.1105/tpc.003947. Plant Cell. 2002. PMID: 12215512 Free PMC article.
-
Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness.J Exp Bot. 2007;58(8):2079-89. doi: 10.1093/jxb/erm074. Epub 2007 Apr 29. J Exp Bot. 2007. PMID: 17470442
-
Over-expression of AtEXLA2 alters etiolated arabidopsis hypocotyl growth.Ann Bot. 2015 Jan;115(1):67-80. doi: 10.1093/aob/mcu221. Epub 2014 Dec 8. Ann Bot. 2015. PMID: 25492062 Free PMC article.
-
Nanoscale structure, mechanics and growth of epidermal cell walls.Curr Opin Plant Biol. 2018 Dec;46:77-86. doi: 10.1016/j.pbi.2018.07.016. Epub 2018 Aug 22. Curr Opin Plant Biol. 2018. PMID: 30142487 Review.
-
Establishing and maintaining axial growth: wall mechanical properties and the cytoskeleton.J Plant Res. 2006 Jan;119(1):5-10. doi: 10.1007/s10265-005-0233-3. Epub 2005 Nov 12. J Plant Res. 2006. PMID: 16284708 Review.
Cited by
-
SHORT-ROOT Controls Cell Elongation in the Etiolated Arabidopsis Hypocotyl.Mol Cells. 2022 Apr 30;45(4):243-256. doi: 10.14348/molcells.2021.5008. Mol Cells. 2022. PMID: 35249891 Free PMC article.
-
Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk.Planta. 2017 Mar;245(3):467-489. doi: 10.1007/s00425-017-2651-6. Epub 2017 Feb 10. Planta. 2017. PMID: 28188422 Review.
-
Microtubule Array Patterns Have a Common Underlying Architecture in Hypocotyl Cells.Plant Physiol. 2018 Jan;176(1):307-325. doi: 10.1104/pp.17.01112. Epub 2017 Sep 11. Plant Physiol. 2018. PMID: 28894021 Free PMC article.
-
Small molecule probes for plant cell wall polysaccharide imaging.Front Plant Sci. 2012 May 11;3:89. doi: 10.3389/fpls.2012.00089. eCollection 2012. Front Plant Sci. 2012. PMID: 22639673 Free PMC article.
-
Organ-specific remodeling of the Arabidopsis transcriptome in response to spaceflight.BMC Plant Biol. 2013 Aug 7;13:112. doi: 10.1186/1471-2229-13-112. BMC Plant Biol. 2013. PMID: 23919896 Free PMC article.
References
-
- Brummell DA, Hall JL (1985) The role of cell wall synthesis in sustained auxin-induced growth. Physiol Plant 63: 406–412
-
- Chanliaud E, Burrows KM, Jeronimidis G, Gidley MJ (2002) Mechanical properties of primary plant cell wall analogues. Planta 215: 989–996 - PubMed
-
- Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50: 391–417 - PubMed
-
- Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

