Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway
- PMID: 15175241
- PMCID: PMC423193
- DOI: 10.1101/gad.1200304
Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway
Abstract
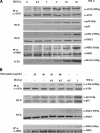
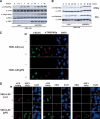
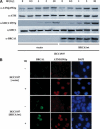
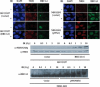
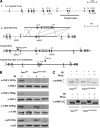
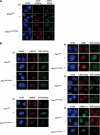
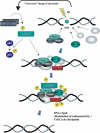
The ATM protein kinase is activated by intermolecular autophosphorylation in response to DNA damage and initiates cellular signaling pathways that facilitate cell survival and reduce chromosomal breakage. Here, we show that NBS1 and BRCA1 are required for the recruitment of previously activated ATM to the sites of DNA breaks after ionizing irradiation, and that this recruitment is required for the phosphorylation of SMC1 by ATM. To explore the functional importance of SMC1 phosphorylation, murine cells were generated, in which the two damage-induced phosphorylation sites in SMC1 are mutated. Although these cells demonstrate normal phosphorylation and focus formation of ATM, NBS1, and BRCA1 proteins after IR, they exhibit a defective S-phase checkpoint, decreased survival, and increased chromosomal aberrations after DNA damage. These observations suggest that many of the abnormal stress responses seen in cells lacking ATM, NBS1, or BRCA1 result from a failure of ATM migration to sites of DNA breaks and a resultant lack of SMC1 phosphorylation.
Figures

Similar articles
-
Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage.Genes Dev. 2002 Mar 1;16(5):560-70. doi: 10.1101/gad.970602. Genes Dev. 2002. PMID: 11877376 Free PMC article.
-
NBS1 prevents chromatid-type aberrations through ATM-dependent interactions with SMC1.Radiat Res. 2008 Sep;170(3):345-52. doi: 10.1667/RR1357.1. Radiat Res. 2008. PMID: 18763866
-
SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint.Genes Dev. 2002 Mar 1;16(5):571-82. doi: 10.1101/gad.970702. Genes Dev. 2002. PMID: 11877377 Free PMC article.
-
The ATM-dependent DNA damage signaling pathway.Cold Spring Harb Symp Quant Biol. 2005;70:99-109. doi: 10.1101/sqb.2005.70.002. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869743 Review.
-
Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review.
Cited by
-
Mitochondrial dysfunction in ataxia-telangiectasia.Blood. 2012 Feb 9;119(6):1490-500. doi: 10.1182/blood-2011-08-373639. Epub 2011 Dec 5. Blood. 2012. PMID: 22144182 Free PMC article.
-
Global gene expression profiling of cells overexpressing SMC3.Mol Cancer. 2005 Sep 12;4:34. doi: 10.1186/1476-4598-4-34. Mol Cancer. 2005. PMID: 16156898 Free PMC article.
-
SMC1A is associated with radioresistance in prostate cancer and acts by regulating epithelial-mesenchymal transition and cancer stem-like properties.Mol Carcinog. 2019 Jan;58(1):113-125. doi: 10.1002/mc.22913. Epub 2018 Oct 5. Mol Carcinog. 2019. PMID: 30242889 Free PMC article.
-
Glioblastoma cells containing mutations in the cohesin component STAG2 are sensitive to PARP inhibition.Mol Cancer Ther. 2014 Mar;13(3):724-32. doi: 10.1158/1535-7163.MCT-13-0749. Epub 2013 Dec 19. Mol Cancer Ther. 2014. PMID: 24356817 Free PMC article.
-
Rapid induction of chromatin-associated DNA mismatch repair proteins after MNNG treatment.DNA Repair (Amst). 2008 Jun 1;7(6):951-69. doi: 10.1016/j.dnarep.2008.03.023. Epub 2008 May 12. DNA Repair (Amst). 2008. PMID: 18468964 Free PMC article.
References
-
- Alligood K.J., Milla, M., Rhodes, N., Ellis, B., Kilpatrick, K.E., Lee, A., Gilmer, T.M., and Lansing, T.J. 2000. Monoclonal antibodies generated against recombinant ATM support kinase activity. Hybridoma 19: 317-321. - PubMed
-
- Bakkenist C.J. and Kastan, M.B. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421: 499-506. - PubMed
-
- Banin S., Moyal, L., Shieh, S., Taya, Y., Anderson, C.W., Chessa, L., Smorodinsky, N.I., Prives, C., Reiss, Y., Shiloh, Y., et al. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281: 1674-1677. - PubMed
-
- Canman C.E., Wolff, A.C., Chen, C.Y., Fornace Jr., A.J., and Kastan, M.B. 1994. The p53-dependent G1 cell cycle checkpoint pathway and ataxia-telangiectasia. Cancer Res. 54: 5054-5058. - PubMed
-
- Canman C.E., Lim, D.S., Cimprich, K.A., Taya, Y., Tamai, K., Sakaguchi, K., Appella, E., Kastan, M.B., and Siliciano, J.D. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281: 1677-1679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
