Global analyses of sumoylated proteins in Saccharomyces cerevisiae. Induction of protein sumoylation by cellular stresses
- PMID: 15166219
- PMCID: PMC2810850
- DOI: 10.1074/jbc.M404173200
Global analyses of sumoylated proteins in Saccharomyces cerevisiae. Induction of protein sumoylation by cellular stresses
Abstract
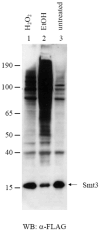
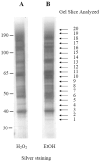
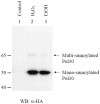
We have undertaken a global analysis of sumoylated proteins in Saccharomyces cerevisiae by tandem mass spectrometry. Exposure of cells to oxidative and ethanol stresses caused large increases in protein sumoylation. A large number of new sumoylated proteins were identified in untreated, hydrogen peroxide-treated, and ethanol-treated cells. These proteins are known to be involved in diverse cellular processes, including gene transcription, protein translation, DNA replication, chromosome segregation, metabolic processes, and stress responses. Additionally, the known enzymes, including E1, E2, and E3 of the sumoylation cascade were found to be auto-sumoylated. Taken together, these results show that protein sumoylation is broadly involved in many cellular functions and this mass spectrometry-based proteomic approach is useful in studying the regulation of protein sumoylation in the cells.
Figures
Similar articles
-
Acute ethanol stress induces sumoylation of conserved chromatin structural proteins in Saccharomyces cerevisiae.Mol Biol Cell. 2021 May 15;32(11):1121-1133. doi: 10.1091/mbc.E20-11-0715. Epub 2021 Mar 31. Mol Biol Cell. 2021. PMID: 33788582 Free PMC article.
-
Sumoylation of the yeast Gcn5 protein.Biochemistry. 2006 Jan 24;45(3):1035-42. doi: 10.1021/bi051624q. Biochemistry. 2006. PMID: 16411780
-
Molecular Circuitry of the SUMO (Small Ubiquitin-like Modifier) Pathway in Controlling Sumoylation Homeostasis and Suppressing Genome Rearrangements.J Biol Chem. 2016 Apr 15;291(16):8825-35. doi: 10.1074/jbc.M116.716399. Epub 2016 Feb 26. J Biol Chem. 2016. PMID: 26921322 Free PMC article.
-
Analysis of Protein Sumoylation.Curr Protoc Protein Sci. 2016 Feb 2;83:14.8.1-14.8.8. doi: 10.1002/0471140864.ps1408s83. Curr Protoc Protein Sci. 2016. PMID: 26836406 Review.
-
SUMO-targeted ubiquitin ligases.Biochim Biophys Acta. 2014 Jan;1843(1):75-85. doi: 10.1016/j.bbamcr.2013.08.022. Epub 2013 Sep 7. Biochim Biophys Acta. 2014. PMID: 24018209 Review.
Cited by
-
Acute ethanol stress induces sumoylation of conserved chromatin structural proteins in Saccharomyces cerevisiae.Mol Biol Cell. 2021 May 15;32(11):1121-1133. doi: 10.1091/mbc.E20-11-0715. Epub 2021 Mar 31. Mol Biol Cell. 2021. PMID: 33788582 Free PMC article.
-
SUMO conjugation regulates immune signalling.Fly (Austin). 2020 Mar-Dec;14(1-4):62-79. doi: 10.1080/19336934.2020.1808402. Epub 2020 Aug 31. Fly (Austin). 2020. PMID: 32777975 Free PMC article. Review.
-
Sumoylation and the structural maintenance of chromosomes (Smc) 5/6 complex slow senescence through recombination intermediate resolution.J Biol Chem. 2010 Apr 16;285(16):11922-30. doi: 10.1074/jbc.M109.041277. Epub 2010 Feb 16. J Biol Chem. 2010. PMID: 20159973 Free PMC article.
-
The 2 microm plasmid causes cell death in Saccharomyces cerevisiae with a mutation in Ulp1 protease.Mol Cell Biol. 2005 May;25(10):4299-310. doi: 10.1128/MCB.25.10.4299-4310.2005. Mol Cell Biol. 2005. PMID: 15870298 Free PMC article.
-
Sumoylation induced by the Arf tumor suppressor: a p53-independent function.Proc Natl Acad Sci U S A. 2005 May 24;102(21):7689-94. doi: 10.1073/pnas.0502978102. Epub 2005 May 16. Proc Natl Acad Sci U S A. 2005. PMID: 15897463 Free PMC article.
References
-
- Hochstrasser M. Annu Rev Genet. 1996;30:405–439. - PubMed
-
- Pickart CM. Annu Rev Biochem. 2001;70:503–533. - PubMed
-
- Weissman AM. Nat Rev Mol Cell Biol. 2001;2:169–178. - PubMed
-
- Melchior F. Annu Rev Cell Dev Biol. 2000;16:591–626. - PubMed
-
- Schwartz DC, Hochstrasser M. Trends Biochem Sci. 2003;28:321–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

