Biodistribution of radioiodinated adenovirus fiber protein knob domain after intravenous injection in mice
- PMID: 15163736
- PMCID: PMC416552
- DOI: 10.1128/JVI.78.12.6431-6438.2004
Biodistribution of radioiodinated adenovirus fiber protein knob domain after intravenous injection in mice
Abstract
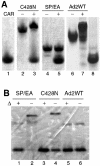
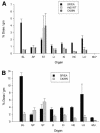
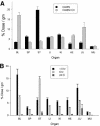
The knob domains from the fiber proteins of adenovirus serotypes 2 and 12 were labeled with radioiodine and then injected into the bloodstreams of mice. Knob proteins with functional binding sites for the coxsackie and adenovirus receptor (CAR) were cleared rapidly from the circulation, with radioactivity appearing predominantly in the stomach, while knob mutants unable to bind to CAR remained in the blood circulation for a prolonged period. The clearance of radiolabeled wild-type knob from the blood was slowed by coinjecting an excess of unlabeled wild-type knob protein. An earlier study showed that (99m)Tc-labeled knob protein with intact CAR-binding activity also cleared rapidly from the blood circulation of mice, with radioactivity accumulating predominantly in the liver (K. R. Zinn et al., Gene Ther. 5:798-808, 1998). Together these results suggest that rapid clearance of knob protein from the blood results from specific binding to CAR in the liver and that the bound knob then enters a degradative pathway. The elevated levels of radioiodine in the stomach observed in our experiments are consistent with deiodination of labeled knob by dehalogenases in hepatocyte microsomes and uptake of the resultant free radioiodine by Na/I symporters in the gastric mucosa. Although CAR has been shown to localize in tight junctions of polarized epithelial cells, where it functions in intercellular adhesion, the results of our study suggest that a subset of CAR molecules in the liver is highly accessible to ligands in the blood and able to rapidly deliver bound ligand to an intracellular degradative compartment.
Figures

Similar articles
-
Adenovirus type 9 fiber knob binds to the coxsackie B virus-adenovirus receptor (CAR) with lower affinity than fiber knobs of other CAR-binding adenovirus serotypes.J Virol. 2001 Aug;75(15):7210-4. doi: 10.1128/JVI.75.15.7210-7214.2001. J Virol. 2001. PMID: 11435605 Free PMC article.
-
Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions.J Virol. 2011 Jul;85(13):6390-402. doi: 10.1128/JVI.00514-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525338 Free PMC article.
-
A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism.Virology. 2003 May 10;309(2):282-93. doi: 10.1016/s0042-6822(03)00067-9. Virology. 2003. PMID: 12758175
-
Adenovirus interaction with its cellular receptor CAR.Curr Top Microbiol Immunol. 2003;272:331-64. doi: 10.1007/978-3-662-05597-7_11. Curr Top Microbiol Immunol. 2003. PMID: 12747555 Review.
-
What does it take to bind CAR?Mol Ther. 2005 Oct;12(4):599-609. doi: 10.1016/j.ymthe.2005.05.017. Mol Ther. 2005. PMID: 16109509 Review.
Cited by
-
The IgCAM CAR Regulates Gap Junction-Mediated Coupling on Embryonic Cardiomyocytes and Affects Their Beating Frequency.Life (Basel). 2022 Dec 21;13(1):14. doi: 10.3390/life13010014. Life (Basel). 2022. PMID: 36675963 Free PMC article.
-
The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells.J Neurosci. 2010 Feb 24;30(8):2897-910. doi: 10.1523/JNEUROSCI.5725-09.2010. J Neurosci. 2010. PMID: 20181587 Free PMC article.
-
Adenovirus Biodistribution is Modified in Sensitive Animals Compared to Naïve Animals.Mol Biotechnol. 2020 Apr;62(4):260-272. doi: 10.1007/s12033-020-00247-x. Mol Biotechnol. 2020. PMID: 32144553
-
Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini.J Virol. 2006 Dec;80(23):11833-51. doi: 10.1128/JVI.00857-06. Epub 2006 Sep 20. J Virol. 2006. PMID: 16987972 Free PMC article.
-
Diversity within the adenovirus fiber knob hypervariable loops influences primary receptor interactions.Nat Commun. 2019 Feb 14;10(1):741. doi: 10.1038/s41467-019-08599-y. Nat Commun. 2019. PMID: 30765704 Free PMC article.
References
-
- Bergelson, J. M. 2003. Virus interactions with mucosal surfaces: alternative receptors, alternative pathways. Curr. Opin. Microbiol. 6:386-391. - PubMed
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. - PubMed
-
- Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

