Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain
- PMID: 15163728
- PMCID: PMC416536
- DOI: 10.1128/JVI.78.12.6344-6359.2004
Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain
Abstract
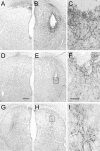
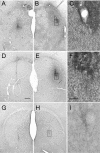
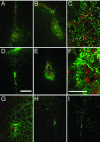
Epidemiological studies report that 80% of the population maintains antibodies (Ab) to wild-type (wt) adeno-associated virus type 2 (AAV2), with 30% expressing neutralizing Ab (NAb). The blood-brain barrier (BBB) provides limited immune privilege to brain parenchyma, and the immune response to recombinant AAV (rAAV) administration in the brain of a naive animal is minimal. However, central nervous system transduction in preimmunized animals remains unstudied. Vector administration may disrupt the BBB sufficiently to promote an immune response in a previously immunized animal. We tested the hypothesis that intracerebral rAAV administration and readministration would not be affected by the presence of circulating Ab to wt AAV2. Rats peripherally immunized with live wt AAV2 and naive controls were tested with single intrastriatal injections of rAAV2 encoding human glial cell line-derived neurotrophic factor (GDNF) or green fluorescent protein (GFP). Striatal readministration of rAAV2-GDNF was also tested in preimmunized and naive rats. Finally, serotype specificity of the immunization against wt AAV2 was examined by single injections of rAAV5-GFP. Preimmunization resulted in high levels of circulating NAb and prevented transduction by rAAV2 as assessed by striatal GDNF levels. rAAV2-GFP striatal transduction was also prevented by immunization, while rAAV5-GFP-mediated transduction, as assessed by stereological cell counting, was unaffected. Additionally, inflammatory markers were present in those animals that received repeated administrations of rAAV2, including markers of a cell-mediated immune response and cytotoxic damage. A live virus immunization protocol generated the circulating anti-wt-AAV Ab seen in this experiment, while human titers are commonly acquired via natural infection. Regardless, the data show that the presence of high levels of NAb against wt AAV can reduce rAAV-mediated transduction in the brain and should be accounted for in future experiments utilizing this vector.
Figures

Similar articles
-
Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented.Mol Ther. 2009 Mar;17(3):524-37. doi: 10.1038/mt.2008.284. Epub 2009 Jan 13. Mol Ther. 2009. PMID: 19142181 Free PMC article.
-
Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson's disease.J Gene Med. 2014 Sep-Oct;16(9-10):300-8. doi: 10.1002/jgm.2779. J Gene Med. 2014. PMID: 25303717
-
Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice.Gene Ther. 2006 Sep;13(17):1300-8. doi: 10.1038/sj.gt.3302766. Epub 2006 May 11. Gene Ther. 2006. PMID: 16688207
-
Recombinant AAV-mediated gene delivery to the central nervous system.J Gene Med. 2004 Feb;6 Suppl 1:S212-22. doi: 10.1002/jgm.506. J Gene Med. 2004. PMID: 14978764 Review.
-
Immune responses to adeno-associated virus and its recombinant vectors.Gene Ther. 2003 Jun;10(11):964-76. doi: 10.1038/sj.gt.3302039. Gene Ther. 2003. PMID: 12756417 Review.
Cited by
-
Current prospects and challenges for epilepsy gene therapy.Exp Neurol. 2013 Jun;244:27-35. doi: 10.1016/j.expneurol.2011.10.003. Epub 2011 Oct 8. Exp Neurol. 2013. PMID: 22008258 Free PMC article. Review.
-
Therapeutic Effect of Platelet-Rich Plasma in Rat Spinal Cord Injuries.Front Neurosci. 2018 Apr 23;12:252. doi: 10.3389/fnins.2018.00252. eCollection 2018. Front Neurosci. 2018. PMID: 29740270 Free PMC article.
-
The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice.J Clin Invest. 2009 Aug;119(8):2388-98. doi: 10.1172/JCI37607. Epub 2009 Jul 1. J Clin Invest. 2009. PMID: 19587448 Free PMC article.
-
Gene and Cell-Based Therapies for Parkinson's Disease: Where Are We?Neurotherapeutics. 2020 Oct;17(4):1539-1562. doi: 10.1007/s13311-020-00940-4. Epub 2020 Oct 30. Neurotherapeutics. 2020. PMID: 33128174 Free PMC article. Review.
-
A next step in adeno-associated virus-mediated gene therapy for neurological diseases: regulation and targeting.Br J Clin Pharmacol. 2013 Aug;76(2):217-32. doi: 10.1111/bcp.12065. Br J Clin Pharmacol. 2013. PMID: 23331189 Free PMC article. Review.
References
-
- Blacklow, N. R., M. D. Hoggan, and W. P. Rowe. 1968. Serologic evidence for human infection with adenovirus-associated viruses. J. Natl. Cancer Inst. 40:319-327. - PubMed
-
- Blacklow, N. R., M. D. Hoggan, M. S. Sereno, C. D. Brandt, H. W. Kim, R. H. Parrott, and R. M. Chanock. 1971. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am. J. Epidemiol. 94:359-366. - PubMed
-
- Brockstedt, D. G., G. M. Podsakoff, L. Fong, G. Kurtzman, W. Mueller-Ruchholtz, and E. G. Engleman. 1999. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 92:67-75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

