Keratinocytes from patients lacking collagen XVII display a migratory phenotype
- PMID: 15161638
- PMCID: PMC1615787
- DOI: 10.1016/S0002-9440(10)63762-5
Keratinocytes from patients lacking collagen XVII display a migratory phenotype
Abstract
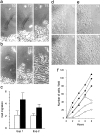
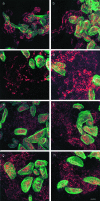
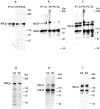
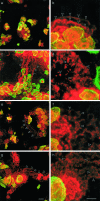
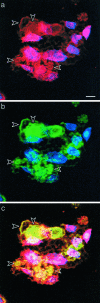
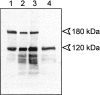
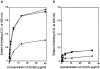
Acquired or inherited junctional epidermolysis bullosa are skin diseases characterized by a separation between the epidermis and the dermis. In inherited nonlethal junctional epidermolysis bullosa, genetic analysis has identified mutations in the COL17A1 gene coding for the transmembrane collagen XVII whereas patients with acquired diseases have autoantibodies against this protein. This suggests that collagen XVII participates in the adhesion of basal keratinocytes to the extracellular matrix. To test this hypothesis, we studied the behavior of keratinocytes with null mutations in the COL17A1 gene. Initial adhesion of mutant cells to laminin 5 was comparable to controls and similarly dependent on alpha3beta1 integrins. The spreading of mutant cells was, however, enhanced, suggesting a propensity to migrate, which was confirmed by migration assays. In addition, laminin 5 deposited by collagen XVII-deficient keratinocytes was scattered and poorly organized, suggesting that correct integration of laminin 5 within the matrix requires collagen XVII. This assumption was supported by the co-distribution of the two proteins in the matrix of normal human keratinocytes and by protein-protein-binding assays showing that the C-terminus of collagen XVII binds to laminin 5. Together, the results unravel an unexpected role of collagen XVII in the regulation of keratinocyte migration.
Figures


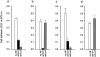


Similar articles
-
Amino acid substitution in the C-terminal domain of collagen XVII reduces laminin-332 interaction causing mild skin fragility with atrophic scarring.Matrix Biol. 2019 Jul;80:72-84. doi: 10.1016/j.matbio.2018.10.003. Epub 2018 Oct 12. Matrix Biol. 2019. PMID: 30316981
-
Deletion of the cytoplasmatic domain of BP180/collagen XVII causes a phenotype with predominant features of epidermolysis bullosa simplex.J Invest Dermatol. 2002 Jan;118(1):185-92. doi: 10.1046/j.0022-202x.2001.01617.x. J Invest Dermatol. 2002. PMID: 11851893
-
Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion--reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes.Exp Cell Res. 1998 Mar 15;239(2):463-76. doi: 10.1006/excr.1997.3923. Exp Cell Res. 1998. PMID: 9521865
-
Generalized atrophic benign epidermolysis bullosa.Adv Dermatol. 1997;13:87-119; discussion 120. Adv Dermatol. 1997. PMID: 9551142 Review.
-
Type XVII collagen gene mutations in junctional epidermolysis bullosa and prospects for gene therapy.Clin Exp Dermatol. 2003 Jan;28(1):53-60. doi: 10.1046/j.1365-2230.2003.01192.x. Clin Exp Dermatol. 2003. PMID: 12558632 Review.
Cited by
-
Bullous Pemphigoid IgG Induces Cell Dysfunction and Enhances the Motility of Epidermal Keratinocytes via Rac1/Proteasome Activation.Front Immunol. 2019 Feb 12;10:200. doi: 10.3389/fimmu.2019.00200. eCollection 2019. Front Immunol. 2019. PMID: 30809225 Free PMC article.
-
Shedding of collagen XVII/BP180 in skin depends on both ADAM10 and ADAM9.J Biol Chem. 2009 Aug 28;284(35):23386-96. doi: 10.1074/jbc.M109.034090. Epub 2009 Jul 1. J Biol Chem. 2009. PMID: 19574220 Free PMC article.
-
From Molecular Insights to Clinical Perspectives in Drug-Associated Bullous Pemphigoid.Int J Mol Sci. 2023 Nov 26;24(23):16786. doi: 10.3390/ijms242316786. Int J Mol Sci. 2023. PMID: 38069109 Free PMC article. Review.
-
Coiled coils ensure the physiological ectodomain shedding of collagen XVII.J Biol Chem. 2012 Aug 24;287(35):29940-8. doi: 10.1074/jbc.M112.345454. Epub 2012 Jul 3. J Biol Chem. 2012. PMID: 22761443 Free PMC article.
-
A Review of Acquired Autoimmune Blistering Diseases in Inherited Epidermolysis Bullosa: Implications for the Future of Gene Therapy.Antibodies (Basel). 2021 May 17;10(2):19. doi: 10.3390/antib10020019. Antibodies (Basel). 2021. PMID: 34067512 Free PMC article. Review.
References
-
- Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. - PubMed
-
- Schäcke H, Schumann H, Hammami-Hauasli N, Raghunath R, Bruckner-Tuderman L. Two forms of collagen XVII in keratinocytes. A full-length transmembrane protein and a soluble ectodomain. J Biol Chem. 1998;273:25937–25943. - PubMed
-
- Hirako Y, Usukura J, Uematsu U, Hashimoto T, Kitajima Y, Owaribe K. Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem. 1998;273:9711–9717. - PubMed
-
- Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin α6β4 important for hemidesmosome assembly. J Cell Sci. 2003;116:387–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

