Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies
- PMID: 15155635
- PMCID: PMC415672
- DOI: 10.1128/IAI.72.6.3315-3324.2004
Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies
Abstract
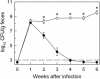
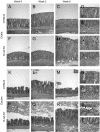
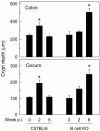
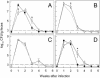
Citrobacter rodentium, a murine model pathogen for human enteropathogenic Escherichia coli, predominantly colonizes the lumen and mucosal surface of the colon and cecum and causes crypt hyperplasia and mucosal inflammation. Mice infected with C. rodentium develop a secretory immunoglobulin A (IgA) response, but the role of B cells or secretory antibodies in host defense is unknown. To address this question, we conducted oral C. rodentium infections in mice lacking B cells, IgA, secreted IgM, polymeric Ig receptor (pIgR), or J chain. Normal mice showed peak bacterial numbers in colon and feces at 1 week and bacterial eradication after 3 to 4 weeks. B-cell-deficient mice were equally susceptible initially but could not control infection subsequently. Tissue responses showed marked differences, as infection of normal mice was accompanied by transient crypt hyperplasia and mucosal inflammation in the colon and cecum at 2 but not 6 weeks, whereas B-cell-deficient mice had few mucosal changes at 2 weeks but severe epithelial hyperplasia with ulcerations and mucosal inflammation at 6 weeks. The functions of B cells were not mediated by secretory antibodies, since mice lacking IgA or secreted IgM or proteins required for their transport into the lumen, pIgR or J chain, cleared C. rodentium normally. Nonetheless, systemic administration of immune sera reduced bacterial numbers significantly in normal and pIgR-deficient mice, and depletion of IgG abrogated this effect. These results indicate that host defense against C. rodentium depends on B cells and IgG antibodies but does not require production or transepithelial transport of IgA or secreted IgM.
Figures


Similar articles
-
Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen.J Immunol. 2004 Jan 1;172(1):433-41. doi: 10.4049/jimmunol.172.1.433. J Immunol. 2004. PMID: 14688352
-
The p50 subunit of NF-kappaB is critical for in vivo clearance of the noninvasive enteric pathogen Citrobacter rodentium.Infect Immun. 2008 Nov;76(11):4978-88. doi: 10.1128/IAI.00736-08. Epub 2008 Aug 11. Infect Immun. 2008. PMID: 18694964 Free PMC article.
-
Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium.Infect Immun. 2003 Sep;71(9):5077-86. doi: 10.1128/IAI.71.9.5077-5086.2003. Infect Immun. 2003. PMID: 12933850 Free PMC article.
-
Role of J chain in secretory immunoglobulin formation.Scand J Immunol. 2000 Sep;52(3):240-8. doi: 10.1046/j.1365-3083.2000.00790.x. Scand J Immunol. 2000. PMID: 10972899 Review.
-
[Properties of mucosal antibodies].J Soc Biol. 2001;195(2):119-24. J Soc Biol. 2001. PMID: 11723823 Review. French.
Cited by
-
TLR3, TRIF, and caspase 8 determine double-stranded RNA-induced epithelial cell death and survival in vivo.J Immunol. 2013 Jan 1;190(1):418-27. doi: 10.4049/jimmunol.1202756. Epub 2012 Dec 3. J Immunol. 2013. PMID: 23209324 Free PMC article.
-
Mucosal IgG in inflammatory bowel disease - a question of (sub)class?Gut Microbes. 2020 Nov 9;12(1):1-9. doi: 10.1080/19490976.2019.1651596. Epub 2019 Sep 3. Gut Microbes. 2020. PMID: 31480888 Free PMC article.
-
Intestinal mucosal responses to microbial infection.Springer Semin Immunopathol. 2005 Sep;27(2):181-96. doi: 10.1007/s00281-005-0207-5. Epub 2005 Jun 1. Springer Semin Immunopathol. 2005. PMID: 15928914 Review.
-
The unexpected link between infection-induced apoptosis and a TH17 immune response.J Leukoc Biol. 2011 Apr;89(4):565-76. doi: 10.1189/jlb.0710421. Epub 2011 Jan 19. J Leukoc Biol. 2011. PMID: 21248151 Free PMC article. Review.
-
A balanced IL-1β activity is required for host response to Citrobacter rodentium infection.PLoS One. 2013 Dec 2;8(12):e80656. doi: 10.1371/journal.pone.0080656. eCollection 2013. PLoS One. 2013. PMID: 24312491 Free PMC article.
References
-
- Barthold, S. W., G. L. Coleman, P. N. Bhatt, G. W. Osbaldiston, and A. M. Jonas. 1976. The etiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 26:889-894. - PubMed
-
- Barthold, S. W., G. L. Coleman, R. O. Jacoby, E. M. Livestone, and A. M. Jonas. 1978. Transmissible murine colonic hyperplasia. Vet. Pathol. 15:223-236. - PubMed
-
- Boes, M., C. Esau, M. B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776-4787. - PubMed
-
- Eckmann, L., F. Laurent, T. D. Langford, M. L. Hetsko, J. R. Smith, M. F. Kagnoff, and F. D. Gillin. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 164:1478-1487. - PubMed
-
- Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. A. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64:5315-5325. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

