HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus
- PMID: 15144898
- PMCID: PMC7111005
- DOI: 10.1016/j.bbrc.2004.04.083
HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus
Abstract
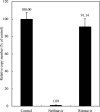
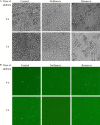
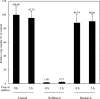
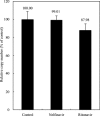
A novel coronavirus has been identified as an etiological agent of severe acute respiratory syndrome (SARS). To rapidly identify anti-SARS drugs available for clinical use, we screened a set of compounds that included antiviral drugs already in wide use. Here we report that the HIV-1 protease inhibitor, nelfinavir, strongly inhibited replication of the SARS coronavirus (SARS-CoV). Nelfinavir inhibited the cytopathic effect induced by SARS-CoV infection. Expression of viral antigens was much lower in infected cells treated with nelfinavir than in untreated infected cells. Quantitative RT-PCR analysis showed that nelfinavir could decrease the production of virions from Vero cells. Experiments with various timings of drug addition revealed that nelfinavir exerted its effect not at the entry step, but at the post-entry step of SARS-CoV infection. Our results suggest that nelfinavir should be examined clinically for the treatment of SARS and has potential as a good lead compound for designing anti-SARS drugs.
Figures

Similar articles
-
The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections.J Med Virol. 2020 Oct;92(10):2087-2095. doi: 10.1002/jmv.25985. Epub 2020 May 17. J Med Virol. 2020. PMID: 32374457 Free PMC article.
-
Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro.J Virol. 2005 Jun;79(11):7095-103. doi: 10.1128/JVI.79.11.7095-7103.2005. J Virol. 2005. PMID: 15890949 Free PMC article.
-
Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice.Antivir Chem Chemother. 2006;17(5):275-84. doi: 10.1177/095632020601700505. Antivir Chem Chemother. 2006. PMID: 17176632
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review.
Cited by
-
Breaking the Chain: Protease Inhibitors as Game Changers in Respiratory Viruses Management.Int J Mol Sci. 2024 Jul 25;25(15):8105. doi: 10.3390/ijms25158105. Int J Mol Sci. 2024. PMID: 39125676 Free PMC article. Review.
-
Quinazolines and thiazolidine-2,4-dions as SARS-CoV-2 inhibitors: repurposing, in silico molecular docking and dynamics simulation.RSC Adv. 2024 Apr 23;14(19):13237-13250. doi: 10.1039/d4ra02029d. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38655479 Free PMC article.
-
Potential Therapeutic Options for COVID-19.Infect Microbes Dis. 2020 Jul 16;2(3):89-95. doi: 10.1097/IM9.0000000000000033. eCollection 2020 Sep. Infect Microbes Dis. 2020. PMID: 38630098 Free PMC article. Review.
-
Structure and function of SARS-CoV and SARS-CoV-2 main proteases and their inhibition: A comprehensive review.Eur J Med Chem. 2023 Nov 15;260:115772. doi: 10.1016/j.ejmech.2023.115772. Epub 2023 Aug 28. Eur J Med Chem. 2023. PMID: 37659195 Review.
-
Recent Advances in Pharmaceutical Approaches of Antimicrobial Agents for Selective Delivery in Various Administration Routes.Antibiotics (Basel). 2023 Apr 27;12(5):822. doi: 10.3390/antibiotics12050822. Antibiotics (Basel). 2023. PMID: 37237725 Free PMC article. Review.
References
-
- Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt G.M, Ahuja A, Yung M.Y, Leung C.B, To K.F, Lui S.F, Szeto C.C, Chung S, Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. - PubMed
-
- S. M. Poutanen, D.E. Low, B. Henry, S. Finkelstein, D. Rose, K. Green, R. Tellier, R. Draker, D. Adachi, M. Ayers, A.K. Chan, D.M. Skowronski, I. Salit, A.E. Simor, A.S. Slutsky, P.W. Doyle, M. Krajden, M. Petric, R.C. Brunham, A.J. McGeer, C. National Microbiology Laboratory, T. Canadian Severe Acute Respiratory Syndrome Study, Identification of severe acute respiratory syndrome in Canada, N. Engl. J. Med. 348 (2003) 1995–2005 - PubMed
-
- Tsang K.W, Ho P.L, Ooi G.C, Yee W.K, Wang T, Chan-Yeung M, Lam W.K, Seto W.H, Yam L.Y, Cheung T.M, Wong P.C, Lam B, Ip M.S, Chan J, Yuen K.Y, Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. - PubMed
-
- WHO, in, 2003
-
- Donnelly C.A, Ghani A.C, Leung G.M, Hedley A.J, Fraser C, Riley S, Abu-Raddad L.J, Ho L.M, Thach T.Q, Chau P, Chan K.P, Lam T.H, Tse L.Y, Tsang T, Liu S.H, Kong J.H, Lau E.M, Ferguson N.M, Anderson R.M. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

