Increased sensitivity to CD4 binding site-directed neutralization following in vitro propagation on primary lymphocytes of a neutralization-resistant human immunodeficiency virus IIIB strain isolated from an accidentally infected laboratory worker
- PMID: 15140962
- PMCID: PMC415840
- DOI: 10.1128/JVI.78.11.5651-5657.2004
Increased sensitivity to CD4 binding site-directed neutralization following in vitro propagation on primary lymphocytes of a neutralization-resistant human immunodeficiency virus IIIB strain isolated from an accidentally infected laboratory worker
Abstract
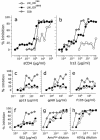
We previously described the adaptation of the neutralization-sensitive human immunodeficiency virus type 1 (HIV-1) strain IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker. During long-term propagation of this resistant isolate, designated FF3346, on primary peripheral blood leukocytes in vitro, an HIV-1 variant appeared that had regained sensitivity to neutralization by soluble CD4 (sCD4) and the broadly neutralizing monoclonal antibody b12. When an early passage of FF3346 was subjected to limiting-dilution culture in peripheral blood mononuclear cells, eight virus variants with various degrees of neutralization resistance were isolated. Two of them, the sCD4 neutralization-resistant variant LW_H8(res) and the sCD4 neutralization-sensitive variant LW_G9(sens), were selected for further study. Interestingly, these two viruses were equally resistant to neutralization by agents that recognize domains other than the CD4 binding site. Site-directed mutagenesis revealed that the increased neutralization sensitivity of variant LW_G9(sens) resulted from only two changes, an Asn-to-Ser substitution at position 164 in the V2 loop and an Ala-to-Glu substitution at position 370 in the C3 domain of gp120. In agreement with this notion, the affinity of b12 for monomeric gp120 containing the N164S and A370E substitutions in the background of the molecular clone LW_H8(res) was higher than its affinity for the parental gp120. Surprisingly, no correlation was observed between CD4 binding affinity for monomeric gp120 and the level of neutralization resistance, suggesting that differences in sCD4 neutralization sensitivity between these viruses are only manifested in the context of the tertiary or quaternary structure of gp120 on the viral surface. The results obtained here indicate that the neutralization-sensitive strain IIIB can become neutralization resistant in vivo under selective pressure by neutralizing antibodies but that this resistance may be easily reversed in the absence of immunological pressure.
Figures


Similar articles
-
The prolonged culture of human immunodeficiency virus type 1 in primary lymphocytes increases its sensitivity to neutralization by soluble CD4.Virology. 2004 Mar 30;321(1):8-22. doi: 10.1016/j.virol.2003.12.012. Virology. 2004. PMID: 15033560
-
Evidence for rapid selection and deletion of HIV-1 subpopulations in vivo by V3-specific neutralizing antibody: a model of humoral-associated selection.Dev Biol Stand. 1990;72:315-41. Dev Biol Stand. 1990. PMID: 2282990
-
Amino acid substitutions in the V3 loop are responsible for adaptation to growth in transformed T-cell lines of a primary human immunodeficiency virus type 1.Virology. 1995 Jul 10;210(2):490-4. doi: 10.1006/viro.1995.1367. Virology. 1995. PMID: 7618285
-
Chimeric viruses expressing primary envelope glycoproteins of human immunodeficiency virus type I show increased sensitivity to neutralization by human sera.Virology. 1996 Jun 15;220(2):450-60. doi: 10.1006/viro.1996.0332. Virology. 1996. PMID: 8661395
-
Relative resistance of primary HIV-1 isolates to neutralization by soluble CD4.Am J Med. 1991 Apr 10;90(4A):22S-26S. doi: 10.1016/0002-9343(91)90407-o. Am J Med. 1991. PMID: 2018048 Review.
Cited by
-
Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies.J Virol. 2004 Dec;78(23):13232-52. doi: 10.1128/JVI.78.23.13232-13252.2004. J Virol. 2004. PMID: 15542675 Free PMC article.
-
Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment.J Virol. 2009 Nov;83(21):10892-907. doi: 10.1128/JVI.01142-09. Epub 2009 Aug 19. J Virol. 2009. PMID: 19692465 Free PMC article.
-
Rapid escape from preserved cross-reactive neutralizing humoral immunity without loss of viral fitness in HIV-1-infected progressors and long-term nonprogressors.J Virol. 2010 Apr;84(7):3576-85. doi: 10.1128/JVI.02622-09. Epub 2010 Jan 13. J Virol. 2010. PMID: 20071586 Free PMC article.
-
Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization.PLoS Pathog. 2016 Jul 19;12(7):e1005742. doi: 10.1371/journal.ppat.1005742. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27434311 Free PMC article.
-
Virus isolates during acute and chronic human immunodeficiency virus type 1 infection show distinct patterns of sensitivity to entry inhibitors.J Virol. 2005 Jul;79(13):8454-69. doi: 10.1128/JVI.79.13.8454-8469.2005. J Virol. 2005. PMID: 15956589 Free PMC article.
References
-
- Beaumont, T., A. van Nuenen, S. Broersen, W. A. Blattner, V. V. Lukashov, and H. Schuitemaker. 2001. Reversal of human immunodeficiency virus type 1 IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J. Virol. 75:2246-2252. - PMC - PubMed
-
- Blaak, H., M. Brouwer, L. J. Ran, F. de Wolf, and H. Schuitemaker. 1998. In vitro replication kinetics of human immunodeficiency virus type 1 (HIV-1) variants in relation to virus load in long-term survivors of HIV-1 infection. J. Infect. Dis. 177:600-610. - PubMed
-
- Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. - PubMed
-
- Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. - PubMed
-
- Cecilia, D., C. Kleeberger, A. Munoz, J. V. Giorgi, and S. Zolla-Pazner. 1999. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J. Infect. Dis. 179:1365-1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

