Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice
- PMID: 15124016
- PMCID: PMC398426
- DOI: 10.1172/JCI19628
Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice
Abstract
Recent evidence indicates that vascular progenitor cells may be the source of smooth muscle cells (SMCs) that accumulate in atherosclerotic lesions, but the origin of these progenitor cells is unknown. To explore the possibility of vascular progenitor cells existing in adults, a variety of tissues from ApoE-deficient mice were extensively examined. Immunohistochemical staining revealed that the adventitia in aortic roots harbored large numbers of cells having stem cell markers, e.g., Sca-1(+) (21%), c-kit(+) (9%), CD34(+) (15%), and Flk1(+) cells (4%), but not SSEA-1(+) embryonic stem cells. Explanted cultures of adventitial tissues using stem cell medium displayed a heterogeneous outgrowth, for example, islands of round-shaped cells surrounded by fibroblast-like cell monolayers. Isolated Sca-1(+) cells were able to differentiate into SMCs in response to PDGF-BB stimulation in vitro. When Sca-1(+) cells carrying the LacZ gene were transferred to the adventitial side of vein grafts in ApoE-deficient mice, beta-gal(+) cells were found in atherosclerotic lesions of the intima, and these cells enhanced the development of the lesions. Thus, a large population of vascular progenitor cells existing in the adventitia can differentiate into SMCs that contribute to atherosclerosis. Our findings indicate that ex vivo expansion of these progenitor cells may have implications for cellular, genetic, and tissue engineering approaches to vascular disease.
Figures
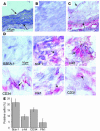

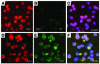

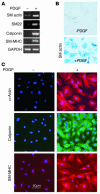
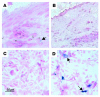

Comment in
-
Lost in transdifferentiation.J Clin Invest. 2004 May;113(9):1249-51. doi: 10.1172/JCI21761. J Clin Invest. 2004. PMID: 15124012 Free PMC article.
Similar articles
-
Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells.Circulation. 2002 Oct 1;106(14):1834-9. doi: 10.1161/01.cir.0000031333.86845.dd. Circulation. 2002. PMID: 12356638
-
Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice.Circ Res. 2003 Oct 17;93(8):e76-86. doi: 10.1161/01.RES.0000097864.24725.60. Epub 2003 Sep 25. Circ Res. 2003. PMID: 14512446
-
Adventitial progenitor cells contribute to arteriosclerosis.Trends Cardiovasc Med. 2005 Feb;15(2):64-8. doi: 10.1016/j.tcm.2005.02.003. Trends Cardiovasc Med. 2005. PMID: 15885572 Review.
-
Proteomic and metabolomic analysis of smooth muscle cells derived from the arterial media and adventitial progenitors of apolipoprotein E-deficient mice.Circ Res. 2008 May 9;102(9):1046-56. doi: 10.1161/CIRCRESAHA.108.174623. Epub 2008 Apr 3. Circ Res. 2008. PMID: 18388323
-
The role of stem cells in atherosclerosis.Arch Mal Coeur Vaiss. 2005 Jun;98(6):672-6. Arch Mal Coeur Vaiss. 2005. PMID: 16007823 Review.
Cited by
-
The adventitia: Essential role in pulmonary vascular remodeling.Compr Physiol. 2011 Jan;1(1):141-61. doi: 10.1002/cphy.c090017. Compr Physiol. 2011. PMID: 23737168 Free PMC article. Review.
-
Adventitia and perivascular cells.Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):e31-5. doi: 10.1161/ATVBAHA.115.306088. Arterioscler Thromb Vasc Biol. 2015. PMID: 26203160 Free PMC article. Review. No abstract available.
-
Hedgehog and Resident Vascular Stem Cell Fate.Stem Cells Int. 2015;2015:468428. doi: 10.1155/2015/468428. Epub 2015 May 6. Stem Cells Int. 2015. PMID: 26064136 Free PMC article. Review.
-
Adventitial Progenitor Cells of Human Great Saphenous Vein Enhance the Resolution of Venous Thrombosis via Neovascularization.Stem Cells Int. 2021 Feb 23;2021:8816763. doi: 10.1155/2021/8816763. eCollection 2021. Stem Cells Int. 2021. PMID: 33679991 Free PMC article.
-
Differentiation of multipotent vascular stem cells contributes to vascular diseases.Nat Commun. 2012 Jun 6;3:875. doi: 10.1038/ncomms1867. Nat Commun. 2012. PMID: 22673902 Free PMC article.
References
-
- Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat. Med. 2001;7:382–383. - PubMed
-
- Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J. Vasc. Res. 2001;38:113–119. - PubMed
-
- Shimizu K, et al. Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat. Med. 2001;7:738–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

