Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts
- PMID: 15121881
- PMCID: PMC452571
- DOI: 10.1091/mbc.e04-02-0103
Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts
Abstract
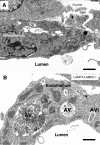
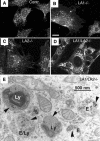
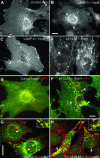
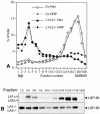
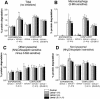
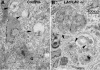
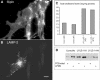
Mice double deficient in LAMP-1 and -2 were generated. The embryos died between embryonic days 14.5 and 16.5. An accumulation of autophagic vacuoles was detected in many tissues including endothelial cells and Schwann cells. Fibroblast cell lines derived from the double-deficient embryos accumulated autophagic vacuoles and the autophagy protein LC3II after amino acid starvation. Lysosomal vesicles were larger and more peripherally distributed and showed a lower specific density in Percoll gradients in double deficient when compared with control cells. Lysosomal enzyme activities, cathepsin D processing and mannose-6-phosphate receptor expression levels were not affected by the deficiency of both LAMPs. Surprisingly, LAMP-1 and -2 deficiencies did not affect long-lived protein degradation rates, including proteolysis due to chaperone-mediated autophagy. The LAMP-1/2 double-deficient cells and, to a lesser extent, LAMP-2 single-deficient cells showed an accumulation of unesterified cholesterol in endo/lysosomal, rab7, and NPC1 positive compartments as well as reduced amounts of lipid droplets. The cholesterol accumulation in LAMP-1/2 double-deficient cells could be rescued by overexpression of murine LAMP-2a, but not by LAMP-1, highlighting the more prominent role of LAMP-2. Taken together these findings indicate partially overlapping functions for LAMP-1 and -2 in lysosome biogenesis, autophagy, and cholesterol homeostasis.
Figures

Similar articles
-
Role of LAMP-2 in lysosome biogenesis and autophagy.Mol Biol Cell. 2002 Sep;13(9):3355-68. doi: 10.1091/mbc.e02-02-0114. Mol Biol Cell. 2002. PMID: 12221139 Free PMC article.
-
Role for Rab7 in maturation of late autophagic vacuoles.J Cell Sci. 2004 Sep 15;117(Pt 20):4837-48. doi: 10.1242/jcs.01370. Epub 2004 Aug 31. J Cell Sci. 2004. PMID: 15340014
-
Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons.J Neurochem. 2002 Dec;83(5):1154-63. doi: 10.1046/j.1471-4159.2002.01220.x. J Neurochem. 2002. PMID: 12437586
-
Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy.Mol Aspects Med. 2006 Oct-Dec;27(5-6):495-502. doi: 10.1016/j.mam.2006.08.005. Epub 2006 Sep 14. Mol Aspects Med. 2006. PMID: 16973206 Review.
-
At the acidic edge: emerging functions for lysosomal membrane proteins.Trends Cell Biol. 2003 Mar;13(3):137-45. doi: 10.1016/s0962-8924(03)00005-9. Trends Cell Biol. 2003. PMID: 12628346 Review.
Cited by
-
Danon disease - dysregulation of autophagy in a multisystem disorder with cardiomyopathy.J Cell Sci. 2016 Jun 1;129(11):2135-43. doi: 10.1242/jcs.184770. Epub 2016 May 10. J Cell Sci. 2016. PMID: 27165304 Free PMC article. Review.
-
A Compendium of Information on the Lysosome.Front Cell Dev Biol. 2021 Dec 15;9:798262. doi: 10.3389/fcell.2021.798262. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977038 Free PMC article. Review.
-
Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death.Mol Brain. 2009 Jul 29;2:24. doi: 10.1186/1756-6606-2-24. Mol Brain. 2009. PMID: 19640278 Free PMC article.
-
The novel RAGE interactor PRAK is associated with autophagy signaling in Alzheimer's disease pathogenesis.Mol Neurodegener. 2016 Jan 12;11:4. doi: 10.1186/s13024-016-0068-5. Mol Neurodegener. 2016. PMID: 26758977 Free PMC article.
-
Glycosylation inhibition reduces cholesterol accumulation in NPC1 protein-deficient cells.Proc Natl Acad Sci U S A. 2015 Dec 1;112(48):14876-81. doi: 10.1073/pnas.1520490112. Epub 2015 Nov 17. Proc Natl Acad Sci U S A. 2015. PMID: 26578804 Free PMC article.
References
-
- Agarraberes, F.A., and Dice, J.F. (2001). A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 114, 2491-2499. - PubMed
-
- Andrejewski, N., Punnonen, E.L., Guhde, G., Tanaka, Y., Lullmann-Rauch, R., Hartmann, D., von Figura, K., and Saftig, P. (1999). Normal lysosomal morphology and function in LAMP-1-deficient mice. J. Biol. Chem. 274, 12692-12701. - PubMed
-
- Aoki, A., and Massa, E.M. (1975). Subcellular compartmentation of free and esterified cholesterol in the interstitial cells of the mouse testis. Cell Tissue Res. 165, 49-62. - PubMed
-
- Brown, M.S., and Goldstein, J.L. (1986). A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34-47. - PubMed
-
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541-1550. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

