Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice
- PMID: 15096611
- PMCID: PMC404098
- DOI: 10.1073/pnas.0401939101
Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice
Abstract
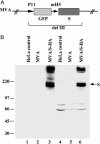
The spike protein (S), a membrane component of severe acute respiratory syndrome coronavirus (SARS-CoV) is anticipated to be an important component of candidate vaccines. We constructed recombinant forms of the highly attenuated modified vaccinia virus Ankara (MVA) containing the gene encoding full-length SARS-CoV S with and without a C-terminal epitope tag called MVA/S-HA and MVA/S, respectively. Cells infected with MVA/Sor MVA/S-HA synthesized a 200-kDa protein, which was recognized by antibody raised against a synthetic peptide of SARS-CoV S or the epitope tag in Western blot analyses. Further studies indicated that S was N-glycosylated and migrated in SDS polyacrylamide gels with an apparent mass of approximately 160 kDa after treatment with peptide N-glycosidase F. The acquisition of resistance to endoglycosidase H indicated trafficking of S to the medial Golgi compartment, and confocal microscopy showed that S was transported to the cell surface. Intranasal or intramuscular inoculations of BALB/c mice with MVA/S produced serum antibodies that recognized the SARS S in ELISA and neutralized SARS-CoV in vitro. Moreover, MVA/S administered by either route elicited protective immunity, as shown by reduced titers of SARS-CoV in the upper and lower respiratory tracts of mice after challenge. Passive transfer of serum from mice immunized with MVA/S to naïve mice also reduced the replication of SARS-CoV in the respiratory tract after challenge, demonstrating a role for antibody to S in protection. The attenuated nature of MVA and the ability of MVA/S to induce neutralizing antibody that protects mice support further development of this candidate vaccine.
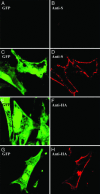
Figures
Similar articles
-
Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region.J Virol. 2005 Mar;79(5):2678-88. doi: 10.1128/JVI.79.5.2678-2688.2005. J Virol. 2005. PMID: 15708987 Free PMC article.
-
Heterologous MVA-S prime Ad5-S boost regimen induces high and persistent levels of neutralizing antibody response against SARS coronavirus.Appl Microbiol Biotechnol. 2007 Oct;76(5):1131-6. doi: 10.1007/s00253-007-1073-y. Epub 2007 Jun 21. Appl Microbiol Biotechnol. 2007. PMID: 17581748 Free PMC article.
-
Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV.Vaccine. 2011 Sep 2;29(38):6606-13. doi: 10.1016/j.vaccine.2011.06.111. Epub 2011 Jul 14. Vaccine. 2011. PMID: 21762752 Free PMC article.
-
The spike protein of SARS-CoV--a target for vaccine and therapeutic development.Nat Rev Microbiol. 2009 Mar;7(3):226-36. doi: 10.1038/nrmicro2090. Epub 2009 Feb 9. Nat Rev Microbiol. 2009. PMID: 19198616 Free PMC article. Review.
-
Vaccine design for severe acute respiratory syndrome coronavirus.Viral Immunol. 2005;18(2):327-32. doi: 10.1089/vim.2005.18.327. Viral Immunol. 2005. PMID: 16035944 Review.
Cited by
-
A systematic review of SARS-CoV-2 vaccine candidates.Signal Transduct Target Ther. 2020 Oct 13;5(1):237. doi: 10.1038/s41392-020-00352-y. Signal Transduct Target Ther. 2020. PMID: 33051445 Free PMC article.
-
Immunization against Medically Important Human Coronaviruses of Public Health Concern.Can J Infect Dis Med Microbiol. 2024 Jun 8;2024:9952803. doi: 10.1155/2024/9952803. eCollection 2024. Can J Infect Dis Med Microbiol. 2024. PMID: 38938549 Free PMC article. Review.
-
Lessons for COVID-19 Immunity from Other Coronavirus Infections.Immunity. 2020 Aug 18;53(2):248-263. doi: 10.1016/j.immuni.2020.07.005. Epub 2020 Jul 14. Immunity. 2020. PMID: 32717182 Free PMC article. Review.
-
Sublingual immunization with recombinant adenovirus encoding SARS-CoV spike protein induces systemic and mucosal immunity without redirection of the virus to the brain.Virol J. 2012 Sep 21;9:215. doi: 10.1186/1743-422X-9-215. Virol J. 2012. PMID: 22995185 Free PMC article.
-
Rectal Acquisition of Simian Immunodeficiency Virus (SIV) SIVmac239 Infection despite Vaccine-Induced Immune Responses against the Entire SIV Proteome.J Virol. 2020 Nov 23;94(24):e00979-20. doi: 10.1128/JVI.00979-20. Print 2020 Nov 23. J Virol. 2020. PMID: 33028714 Free PMC article.
References
-
- Drosten, C., Gunther, S., Preiser, W., van der Werf, S., Brodt, H. R., Becker, S., Rabenau, H., Panning, M., Kolesnikova, L., Fouchier, R. A. et al. (2003) N. Engl. J. Med. 348, 1967-1976. - PubMed
-
- Ksiazek, T. G., Erdman, D., Goldsmith, C. S., Zaki, S. R., Peret, T., Emery, S., Tong, S., Urbani, C., Comer, J. A., Lim, W., et al. (2003) N. Engl. J. Med. 348, 1953-1966. - PubMed
-
- Rota, P. A., Oberste, M. S., Monroe, S. S., Nix, W. A., Campagnoli, R., Icenogle, J. P., Penaranda, S., Bankamp, B., Maher, K., Chen, M. H., et al. (2003) Science 300, 1394-1399. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

