DNA vaccines against human immunodeficiency virus type 1 in the past decade
- PMID: 15084506
- PMCID: PMC387404
- DOI: 10.1128/CMR.17.2.370-389.2004
DNA vaccines against human immunodeficiency virus type 1 in the past decade
Abstract
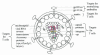
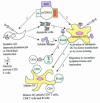
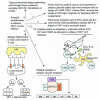
This article reviews advances in the field of human immunodeficiency virus type 1 (HIV-1) and AIDS vaccine development over the last decade, with an emphasis on the DNA vaccination approach. Despite the discovery of HIV-1 and AIDS in humans nearly 20 years ago, there is no vaccine yet that can prevent HIV-1 infection. The focus has shifted toward developing vaccines that can control virus replication and disease progression by eliciting broadly cross-reactive T-cell responses. Among several approaches evaluated, the DNA-based modality has shown considerable promise in terms of its ability to elicit cellular immune responses in primate studies. Of great importance are efforts aimed at improvement of the potency of this modality in the clinic. The review discusses principles of DNA vaccine design and the various mechanisms of plasmid-encoded antigen presentation. The review also outlines current DNA-based vaccine strategies and vectors that have successfully been shown to control virus replication and slow disease progression in animal models. Finally, it lists recent strategies that have been developed as well as novel approaches under consideration to enhance the immunogenicity of plasmid-encoded HIV-1 antigen in various animal models.
Figures

Similar articles
-
DNA prime/MVTT boost regimen with HIV-1 mosaic Gag enhances the potency of antigen-specific immune responses.Vaccine. 2018 Jul 25;36(31):4621-4632. doi: 10.1016/j.vaccine.2018.06.047. Epub 2018 Jun 28. Vaccine. 2018. PMID: 29961605
-
Enhanced immunogenicity of an HIV-1 DNA vaccine delivered with electroporation via combined intramuscular and intradermal routes.J Virol. 2014 Jun;88(12):6959-69. doi: 10.1128/JVI.00183-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719412 Free PMC article.
-
Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques.J Virol. 2007 May;81(10):5257-69. doi: 10.1128/JVI.00055-07. Epub 2007 Feb 28. J Virol. 2007. PMID: 17329330 Free PMC article.
-
Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models.Expert Rev Vaccines. 2017 Oct;16(10):973-985. doi: 10.1080/14760584.2017.1371594. Epub 2017 Sep 4. Expert Rev Vaccines. 2017. PMID: 28838267 Free PMC article. Review.
-
Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1077-88. doi: 10.1089/AID.2015.0101. Epub 2015 Aug 13. AIDS Res Hum Retroviruses. 2015. PMID: 26149745 Review.
Cited by
-
DNA vaccines for HIV: challenges and opportunities.Springer Semin Immunopathol. 2006 Nov;28(3):267-79. doi: 10.1007/s00281-006-0046-z. Epub 2006 Oct 10. Springer Semin Immunopathol. 2006. PMID: 17031649 Review.
-
Use of cytokine immunotherapy to block CNS demyelination induced by a recombinant HSV-1 expressing IL-2.Gene Ther. 2011 Jul;18(7):734-42. doi: 10.1038/gt.2011.32. Epub 2011 Mar 17. Gene Ther. 2011. PMID: 21412284 Free PMC article.
-
Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity.Hum Vaccin Immunother. 2017 Dec 2;13(12):2837-2848. doi: 10.1080/21645515.2017.1330236. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28604157 Free PMC article. Review.
-
DNA and modified vaccinia virus Ankara vaccines encoding multiple cytotoxic and helper T-lymphocyte epitopes of human immunodeficiency virus type 1 (HIV-1) are safe but weakly immunogenic in HIV-1-uninfected, vaccinia virus-naive adults.Clin Vaccine Immunol. 2012 May;19(5):649-58. doi: 10.1128/CVI.00038-12. Epub 2012 Mar 7. Clin Vaccine Immunol. 2012. PMID: 22398243 Free PMC article. Clinical Trial.
-
Vaccination of rhesus macaques with a vif-deleted simian immunodeficiency virus proviral DNA vaccine.Virology. 2008 May 10;374(2):261-72. doi: 10.1016/j.virol.2008.01.020. Epub 2008 Feb 7. Virology. 2008. PMID: 18261756 Free PMC article.
References
-
- Agadjanyan, M. G., J. J. Kim, N. Trivedi, D. M. Wilson, B. Monzavi-Karbassi, L. D. Morrison, L. K. Nottingham, T. Dentchev, A. Tsai, K. Dang, A. A. Chalian, M. A. Maldonado, W. V. Williams, and D. B. Weiner. 1999. CD86 (B7-2) can function to drive MHC-restricted antigen-specific CTL responses in vivo. J. Immunol. 162:3417-3427. - PubMed
-
- Aihara, H., and J. Miyazaki. 1998. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16:867-870. - PubMed
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

