A novel role for shuttling SR proteins in mRNA translation
- PMID: 15082528
- PMCID: PMC387416
- DOI: 10.1101/gad.286404
A novel role for shuttling SR proteins in mRNA translation
Abstract
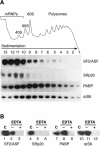
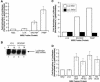
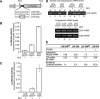
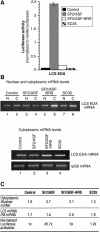
The Ser-Arg-rich (SR) proteins comprise a large family of nuclear phosphoproteins that are required for constitutive and alternative splicing. A subset of SR proteins shuttles continuously between the nucleus and the cytoplasm, suggesting that the role of shuttling SR proteins in gene expression may not be limited to nuclear pre-mRNA splicing, but may also include unknown cytoplasmic functions. Here, we show that shuttling SR proteins, in particular SF2/ASF, associate with translating ribosomes and stimulate translation when tethered to a reporter mRNA in Xenopus oocytes. Moreover, SF2/ASF enhances translation of reporter mRNAs in HeLa cells, and this activity is dependent on its ability to shuttle from the nucleus to the cytoplasm and is increased by the presence of an exonic-splicing enhancer. Furthermore, SF2/ASF can stimulate translation in vitro using a HeLa cell-free translation system. Thus, the association of SR proteins with translating ribosomes, as well as the stimulation of translation both in vivo and in vitro, strongly suggest a role for shuttling SR proteins in translation. We propose that shuttling SR proteins play multiple roles in the posttranscriptional expression of eukaryotic genes and illustrate how they may couple splicing and translation.
Figures



Similar articles
-
Reversible phosphorylation differentially affects nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor.Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):15042-7. doi: 10.1073/pnas.0507827102. Epub 2005 Oct 6. Proc Natl Acad Sci U S A. 2005. PMID: 16210245 Free PMC article.
-
Identification of nuclear and cytoplasmic mRNA targets for the shuttling protein SF2/ASF.PLoS One. 2008 Oct 8;3(10):e3369. doi: 10.1371/journal.pone.0003369. PLoS One. 2008. PMID: 18841201 Free PMC article.
-
A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm.Genes Dev. 1998 Jan 1;12(1):55-66. doi: 10.1101/gad.12.1.55. Genes Dev. 1998. PMID: 9420331 Free PMC article.
-
Shuttling SR proteins: more than splicing factors.FEBS J. 2011 Sep;278(18):3246-55. doi: 10.1111/j.1742-4658.2011.08274.x. Epub 2011 Aug 24. FEBS J. 2011. PMID: 21794093 Review.
-
SR Proteins: Binders, Regulators, and Connectors of RNA.Mol Cells. 2017 Jan;40(1):1-9. doi: 10.14348/molcells.2017.2319. Epub 2017 Jan 26. Mol Cells. 2017. PMID: 28152302 Free PMC article. Review.
Cited by
-
Splicing-factor oncoprotein SRSF1 stabilizes p53 via RPL5 and induces cellular senescence.Mol Cell. 2013 Apr 11;50(1):56-66. doi: 10.1016/j.molcel.2013.02.001. Epub 2013 Mar 7. Mol Cell. 2013. PMID: 23478443 Free PMC article.
-
SRSF1 and SRSF9 RNA binding proteins promote Wnt signalling-mediated tumorigenesis by enhancing β-catenin biosynthesis.EMBO Mol Med. 2013 May;5(5):737-50. doi: 10.1002/emmm.201202218. Epub 2013 Apr 17. EMBO Mol Med. 2013. PMID: 23592547 Free PMC article.
-
The role of splicing factors in deregulation of alternative splicing during oncogenesis and tumor progression.Mol Cell Oncol. 2014 Dec 1;2(1):e970955. doi: 10.4161/23723548.2014.970955. eCollection 2015 Jan-Mar. Mol Cell Oncol. 2014. PMID: 27308389 Free PMC article. Review.
-
SR Splicing Factors Promote Cancer via Multiple Regulatory Mechanisms.Genes (Basel). 2022 Sep 16;13(9):1659. doi: 10.3390/genes13091659. Genes (Basel). 2022. PMID: 36140826 Free PMC article. Review.
-
The RNA polymerase II CTD kinase Ctk1 functions in translation elongation.Genes Dev. 2007 Jun 1;21(11):1409-21. doi: 10.1101/gad.428407. Genes Dev. 2007. PMID: 17545469 Free PMC article.
References
-
- Blencowe B.J. 2000. Exonic splicing enhancers: Mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 25: 106-110. - PubMed
-
- Burge C.B., Padgett, R.A., and Sharp, P.A. 1998. Evolutionary fates and origins of U12-type introns. Mol. Cell 2: 773-785. - PubMed
-
- Caceres J.F. and Kornblihtt, A.R. 2002. Alternative splicing: Multiple control mechanisms and involvement in human disease. Trends Genet. 18: 186-193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
