Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine
- PMID: 15081618
- PMCID: PMC7119895
- DOI: 10.1016/j.imlet.2004.01.001
Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine
Abstract
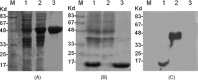
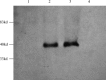
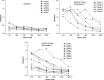
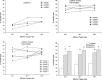
Induction of effective cytotoxic T lymphocyte (CTL) and/or a specific antibody against conserved viral proteins may be essential to the development of a safe and effective severe acute respiratory syndrome coronavirus (SARS-Cov) vaccine. DNA vaccination represents a new strategy for induction of humoral and cellular immune response. To determine the ability of SARS-Cov nucleoprotein (N protein) to induce antiviral immunity, in this report, we established a stable C2C12 line expressing SARS-Cov N protein, which was used as a target for specific CTL assay. We also expressed recombinant N proteins in Escherichia coli and prepared N protein-specific polyclonal antibodies. C3H/He mice were immunized with N protein-expressible pcDN-fn vector by intramuscular injections. We found that the DNA vaccination induced both N protein-specific antibody and specific CTL activity to the target. When C3H/He mice were immunized by three separate injections, high antibody titre (1:3200-1:6400, average titre is 1:4580) and high CTL activity (67.4+/-8.4% (E:T = 25:1), 69.6+/-6.7% (E:T = 50:1) and 71.8+/-6.2% (E:T = 100:1)) were observed. In the case of two vaccine injections, CTL activity was also high (56.6+/-12.7% (E:T = 25:1), 57.4+/-11.7% (E:T = 50:1) and 63.0+/-6.3% (E:T = 100:1)) However, antibody titres were much lower (1:200-1:3200, average titre is 1:980). Our results suggest that SARS-Cov nucleocapsid gene might be a candidate gene for SARS DNA vaccination.
Figures

Similar articles
-
Enhanced induction of SARS-CoV nucleocapsid protein-specific immune response using DNA vaccination followed by adenovirus boosting in BALB/c mice.Intervirology. 2006;49(5):307-18. doi: 10.1159/000094247. Epub 2006 Jun 29. Intervirology. 2006. PMID: 16809936 Free PMC article.
-
Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus.J Gen Virol. 2006 Mar;87(Pt 3):641-650. doi: 10.1099/vir.0.81579-0. J Gen Virol. 2006. PMID: 16476986
-
Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice.Clin Vaccine Immunol. 2007 Jul;14(7):894-901. doi: 10.1128/CVI.00019-07. Epub 2007 May 9. Clin Vaccine Immunol. 2007. PMID: 17494640 Free PMC article.
-
Studies of SARS virus vaccines.Hong Kong Med J. 2008 Aug;14 Suppl 4:39-43. Hong Kong Med J. 2008. PMID: 18708674 Review.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
Mass Spectrometry Analysis of SARS-CoV-2 Nucleocapsid Protein Reveals Camouflaging Glycans and Unique Post-Translational Modifications.Infect Microbes Dis. 2021 Aug 3;3(3):149-157. doi: 10.1097/IM9.0000000000000071. eCollection 2021 Sep. Infect Microbes Dis. 2021. PMID: 38630108 Free PMC article.
-
Combination of Recombinant Proteins S1/N and RBD/N as Potential Vaccine Candidates.Vaccines (Basel). 2023 Apr 18;11(4):864. doi: 10.3390/vaccines11040864. Vaccines (Basel). 2023. PMID: 37112776 Free PMC article.
-
BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus.Vaccines (Basel). 2022 May 4;10(5):721. doi: 10.3390/vaccines10050721. Vaccines (Basel). 2022. PMID: 35632475 Free PMC article.
-
Immune responses to human respiratory coronaviruses infection in mouse models.Curr Opin Virol. 2022 Feb;52:102-111. doi: 10.1016/j.coviro.2021.11.015. Epub 2021 Dec 11. Curr Opin Virol. 2022. PMID: 34906757 Free PMC article. Review.
-
The variants of SARS-CoV-2 and the challenges of vaccines.J Med Virol. 2022 Apr;94(4):1366-1372. doi: 10.1002/jmv.27513. Epub 2021 Dec 15. J Med Virol. 2022. PMID: 34890492 Free PMC article. Review.
References
-
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
-
- Vanderzanden L., Bray M., Fuller D., Roberts T., Custer D., Spik K. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998;246:134–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

