The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase
- PMID: 15073337
- PMCID: PMC395939
- DOI: 10.1073/pnas.0401602101
The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase
Abstract
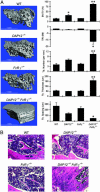
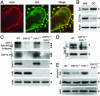
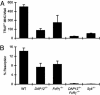
Osteoclasts, the only bone-resorbing cells, are central to the pathogenesis of osteoporosis, yet their development and regulation are incompletely understood. Multiple receptors of the immune system use a common signaling paradigm whereby phosphorylated immunoreceptor tyrosine-based activation motifs (ITAMs) within receptor-associated adapter proteins recruit the Syk tyrosine kinase. Here we demonstrate that a similar mechanism is required for development of functional osteoclasts. Mice lacking two ITAM-bearing adapters, DAP12 and the Fc receptor gamma-chain (FcRgamma), are severely osteopetrotic. DAP12(-/-)FcRgamma(-/-) bone marrow cells fail to differentiate into multinucleated osteoclasts or resorb bone in vitro and show impaired phosphorylation of the Syk tyrosine kinase. syk(-/-) progenitors are similarly defective in osteoclast development and bone resorption. Intact SH2-domains of Syk, introduced by retroviral transduction, are required for functional reconstitution of syk(-/-) osteoclasts, whereas intact ITAM-domains on DAP12 are required for reconstitution of DAP12(-/-) FcRgamma(-/-) cells. These data indicate that recruitment of Syk to phosphorylated ITAMs is critical for osteoclastogenesis. Although DAP12 appears to be primarily responsible for osteoclast differentiation in cultures directly stimulated with macrophage-colony stimulating factor and receptor activator of NF-kappaB ligand cytokines, DAP12 and FcRgamma have overlapping roles in supporting osteoclast development in osteoblast-osteoclast cocultures, which mirrors their overlapping functions in vivo. These results provide new insight into the biology of osteoclasts and suggest novel therapeutic targets in diseases of bony remodeling.
Figures


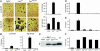
Similar articles
-
High dose M-CSF partially rescues the Dap12-/- osteoclast phenotype.J Cell Biochem. 2003 Dec 1;90(5):871-83. doi: 10.1002/jcb.10694. J Cell Biochem. 2003. PMID: 14624447
-
Absence of Dap12 and the αvβ3 integrin causes severe osteopetrosis.J Cell Biol. 2015 Jan 5;208(1):125-36. doi: 10.1083/jcb.201410123. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547154 Free PMC article.
-
Role of ITAM-containing adapter proteins and their receptors in the immune system and bone.Immunol Rev. 2005 Dec;208:50-65. doi: 10.1111/j.0105-2896.2005.00325.x. Immunol Rev. 2005. PMID: 16313340 Review.
-
[Regulation of osteoclast development by immunoglobulin-like receptors].Nihon Rinsho. 2005 Sep;63(9):1562-8. Nihon Rinsho. 2005. PMID: 16164212 Review. Japanese.
-
DOK3 Modulates Bone Remodeling by Negatively Regulating Osteoclastogenesis and Positively Regulating Osteoblastogenesis.J Bone Miner Res. 2017 Nov;32(11):2207-2218. doi: 10.1002/jbmr.3205. Epub 2017 Aug 2. J Bone Miner Res. 2017. PMID: 28650106 Free PMC article.
Cited by
-
Biological Effects of β-Glucans on Osteoclastogenesis.Molecules. 2021 Apr 1;26(7):1982. doi: 10.3390/molecules26071982. Molecules. 2021. PMID: 33915775 Free PMC article. Review.
-
Tyrosine kinase inhibitors for the treatment of rheumatoid arthritis.Curr Top Med Chem. 2013;13(6):760-73. doi: 10.2174/15680266113139990094. Curr Top Med Chem. 2013. PMID: 23574525 Free PMC article. Review.
-
TULA-2, a novel histidine phosphatase, regulates bone remodeling by modulating osteoclast function.Cell Mol Life Sci. 2013 Apr;70(7):1269-84. doi: 10.1007/s00018-012-1203-2. Epub 2012 Nov 13. Cell Mol Life Sci. 2013. PMID: 23149425 Free PMC article.
-
The transmembrane adapter SCIMP recruits tyrosine kinase Syk to phosphorylate Toll-like receptors to mediate selective inflammatory outputs.J Biol Chem. 2022 May;298(5):101857. doi: 10.1016/j.jbc.2022.101857. Epub 2022 Mar 22. J Biol Chem. 2022. PMID: 35337798 Free PMC article.
-
Osteoclastic differentiation and function regulated by old and new pathways.Rev Endocr Metab Disord. 2006 Jun;7(1-2):23-32. doi: 10.1007/s11154-006-9010-4. Rev Endocr Metab Disord. 2006. PMID: 17115268 Review.
References
-
- Boyle, W. J., Simonet, W. S. & Lacey, D. L. (2003) Nature 423, 337-342. - PubMed
-
- Turner, M., Schweighoffer, E., Colucci, F., Di Santo, J. P. & Tybulewicz, V. L. (2000) Immunol. Today 21, 148-154. - PubMed
-
- Lanier, L. L. & Bakker, A. B. (2000) Immunol. Today 21, 611-614. - PubMed
-
- Takai, T., Li, M., Sylvestre, D., Clynes, R. & Ravetch, J. V. (1994) Cell 76, 519-529. - PubMed
-
- Paloneva, J., Kestila, M., Wu, J., Salminen, A., Bohling, T., Ruotsalainen, V., Hakola, P., Bakker, A. B., Phillips, J. H., Pekkarinen, P., et al. (2000) Nat. Genet. 25, 357-361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

