Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins
- PMID: 15070779
- PMCID: PMC384808
- DOI: 10.1073/pnas.0306924101
Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins
Abstract
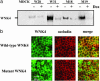
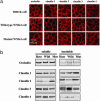
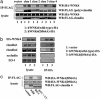
Mutations in the WNK4 gene cause pseudohypoaldosteronism type II (PHAII), an autosomal-dominant disorder of hyperkalemia and hypertension. The target molecules of this putative kinase and the molecular mechanisms by which the mutations cause the phenotypes are currently unknown. Although recent reports found that expression of WNK4 in Xenopus oocytes causes inhibition of the thiazide-sensitive NaCl cotransporter and the renal K channel ROMK, there may be additional targets of WNK4. For example, an increase in paracellular chloride permeability has been postulated to be a mediator of PHAII pathogenesis, a possibility supported by the localization of WNK4 at tight junctions in vivo. To determine the validity of this hypothesis, we measured transepithelial Na and Cl permeability in Madin-Darby canine kidney II cells stably expressing wild-type or a pathogenic mutant of WNK4. We found that transepithelial paracellular Cl permeability was increased in cells expressing a disease-causing mutant WNK4 (D564A) but that Na permeability was decreased slightly. Furthermore, WNK4 bound and phosphorylated claudins 1-4, major tight-junction membrane proteins known to be involved in the regulation of paracellular ion permeability. The increases in phosphorylation of claudins were greater in cells expressing the mutant WNK4 than in cells expressing wild-type protein. These results clearly indicate that the pathogenic WNK4 mutant possesses a gain-of-function activity and that the claudins may be important molecular targets of WNK4 kinase. The increased paracellular "chloride shunt" caused by the mutant WNK4 could be the pathogenic mechanism of PHAII.
Figures
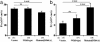

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells.Am J Physiol Regul Integr Comp Physiol. 2008 Nov;295(5):R1713-9. doi: 10.1152/ajpregu.90596.2008. Epub 2008 Sep 10. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18784328 Free PMC article.
-
Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins.Am J Physiol Renal Physiol. 2006 Dec;291(6):F1132-41. doi: 10.1152/ajprenal.00063.2006. Epub 2006 Jun 13. Am J Physiol Renal Physiol. 2006. PMID: 16774906 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
-
Claudins and the kidney.Annu Rev Physiol. 2013;75:479-501. doi: 10.1146/annurev-physiol-030212-183705. Epub 2012 Nov 5. Annu Rev Physiol. 2013. PMID: 23140368 Free PMC article. Review.
-
Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process.Proc Natl Acad Sci U S A. 2012 May 15;109(20):7929-34. doi: 10.1073/pnas.1200947109. Epub 2012 May 1. Proc Natl Acad Sci U S A. 2012. PMID: 22550170 Free PMC article.
-
WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo.Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):4020-4. doi: 10.1073/pnas.0611727104. Epub 2007 Feb 26. Proc Natl Acad Sci U S A. 2007. PMID: 17360470 Free PMC article.
-
WNK1 activates SGK1 to regulate the epithelial sodium channel.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10315-20. doi: 10.1073/pnas.0504422102. Epub 2005 Jul 8. Proc Natl Acad Sci U S A. 2005. PMID: 16006511 Free PMC article.
-
Familial hyperkalemia and hypertension and a hypothesis to explain proximal renal tubular acidosis.Proc Natl Acad Sci U S A. 2019 Aug 13;116(33):16173-16174. doi: 10.1073/pnas.1909494116. Epub 2019 Aug 1. Proc Natl Acad Sci U S A. 2019. PMID: 31371517 Free PMC article. No abstract available.
References
-
- Wilson, F. H., Disse-Nicodeme, S., Choate, K. A., Ishikawa, K., Nelson-Williams, C., Desitter, I., Gunel, M., Milford, D. V., Lipkin, G. W., Achard, J. M., et al. (2001) Science 293, 1107-1112. - PubMed
-
- Schambelan, M., Sebastian, A. & Rector, F. C., Jr. (1981) Kidney Int. 19, 716-727. - PubMed
-
- Farfel, Z., Iaina, A., Rosenthal, T., Waks, U., Shibolet, S. & Gafni, J. (1978) Arch. Intern. Med. (Moscow) 138, 1828-1832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

