Quantitative analysis of the formation and diffusion of A1-adenosine receptor-antagonist complexes in single living cells
- PMID: 15070776
- PMCID: PMC384805
- DOI: 10.1073/pnas.0400420101
Quantitative analysis of the formation and diffusion of A1-adenosine receptor-antagonist complexes in single living cells
Abstract
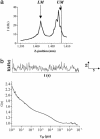
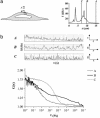
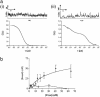
The A1-adenosine receptor (A1-AR) is a G protein-coupled receptor that mediates many of the physiological effects of adenosine in the brain, heart, kidney, and adipocytes. Currently, ligand interactions with the A1-AR can be quantified on large cell populations only by using radioligand binding. To increase the resolution of these measurements, we have designed and characterized a previously undescribed fluorescent antagonist for the A1-AR, XAC-BY630, based on xanthine amine congener (XAC). This compound has been used to quantify ligand-receptor binding at a single cell level using fluorescence correlation spectroscopy (FCS). XAC-BY630 was a competitive antagonist of A1-AR-mediated inhibition of cAMP accumulation [log10 of the affinity constant (pKb) = 6.7)] and stimulation of inositol phosphate accumulation (pKb = 6.5). Specific binding of XAC-BY630 to cell surface A1-AR could also be visualized in living Chinese hamster ovary (CHO)-A1 cells by using confocal microscopy. FCS analysis of XAC-BY630 binding to the membrane of CHO-A1 cells revealed three components with diffusion times (tauD) of 62 micros (tauD1, free ligand), 17 ms (tauD2, A1-AR-ligand), and 320 ms (tauD3). Confirmation that tauD2 resulted from diffusion of ligand-receptor complexes came from the similar diffusion time observed for the fluorescent A1-AR-Topaz fusion protein (15 ms). Quantification of tauD2 showed that the number of receptor-ligand complexes increased with increasing free ligand concentration and was decreased by the selective A1-AR antagonist, 8-cyclopentyl-1,3-dipropylxanthine. The combination of FCS with XAC-BY630 will be a powerful tool for the characterization of ligand-A1-AR interactions in single living cells in health and disease.
Figures
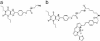


Similar articles
-
Application of fluorescence correlation spectroscopy to the measurement of agonist binding to a G-protein coupled receptor at the single cell level.Faraday Discuss. 2004;126:197-207; discussion 245-54. doi: 10.1039/b307407b. Faraday Discuss. 2004. PMID: 14992407
-
Antagonist selective modulation of adenosine A1 and A3 receptor pharmacology by the food dye Brilliant Black BN: evidence for allosteric interactions.Mol Pharmacol. 2010 Apr;77(4):678-86. doi: 10.1124/mol.109.063065. Epub 2010 Jan 19. Mol Pharmacol. 2010. PMID: 20086038 Free PMC article.
-
Influence of fluorophore and linker composition on the pharmacology of fluorescent adenosine A1 receptor ligands.Br J Pharmacol. 2010 Feb;159(4):772-86. doi: 10.1111/j.1476-5381.2009.00488.x. Epub 2010 Jan 26. Br J Pharmacol. 2010. PMID: 20105183 Free PMC article.
-
Agonist-occupied A3 adenosine receptors exist within heterogeneous complexes in membrane microdomains of individual living cells.FASEB J. 2008 Mar;22(3):850-60. doi: 10.1096/fj.07-8180com. Epub 2007 Oct 24. FASEB J. 2008. PMID: 17959910
-
A1 adenosine receptor inhibition of cyclic AMP formation and radioligand binding in the guinea-pig cerebral cortex.Br J Pharmacol. 1994 Dec;113(4):1501-7. doi: 10.1111/j.1476-5381.1994.tb17166.x. Br J Pharmacol. 1994. PMID: 7889308 Free PMC article.
Cited by
-
Lighting up G protein-coupled purinergic receptors with engineered fluorescent ligands.Neuropharmacology. 2015 Nov;98:58-67. doi: 10.1016/j.neuropharm.2015.04.001. Epub 2015 Apr 16. Neuropharmacology. 2015. PMID: 25890205 Free PMC article. Review.
-
Xanthines as adenosine receptor antagonists.Handb Exp Pharmacol. 2011;(200):151-99. doi: 10.1007/978-3-642-13443-2_6. Handb Exp Pharmacol. 2011. PMID: 20859796 Free PMC article. Review.
-
Nanoparticles as fluorescence labels: is size all that matters?Biophys J. 2008 Jul;95(2):865-76. doi: 10.1529/biophysj.107.127688. Epub 2008 Apr 4. Biophys J. 2008. PMID: 18390610 Free PMC article.
-
Adenosine A(2A) receptor dynamics studied with the novel fluorescent agonist Alexa488-APEC.Eur J Pharmacol. 2008 Aug 20;590(1-3):36-42. doi: 10.1016/j.ejphar.2008.05.036. Epub 2008 Jul 4. Eur J Pharmacol. 2008. PMID: 18603240 Free PMC article.
-
Kinetic analysis of antagonist-occupied adenosine-A3 receptors within membrane microdomains of individual cells provides evidence of receptor dimerization and allosterism.FASEB J. 2014 Oct;28(10):4211-22. doi: 10.1096/fj.13-247270. Epub 2014 Jun 26. FASEB J. 2014. PMID: 24970394 Free PMC article.
References
-
- Ralevic, V. R. & Burnstock, G. (1998) Pharmacol. Rev. 50, 415-475. - PubMed
-
- Klotz, K.-N. (2000) Naunyn-Schmiedeberg's Arch. Pharmacol. 362, 382-391. - PubMed
-
- Cordeaux, Y., Briddon, S. J., Megson, A. E., McDonnell, J., Dickenson, J. M. & Hill, S. J. (2001) Mol. Pharmacol. 58, 1075-1084. - PubMed
-
- Gines, S., Ciruela, F., Burgueno, J., Casado, V., Canela, E. I., Mallol, J., Lluis, C. & Franco, R. (2001) Mol. Pharmacol. 59, 1314-1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

