M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP
- PMID: 15070733
- PMCID: PMC384762
- DOI: 10.1073/pnas.0307700101
M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP
Abstract
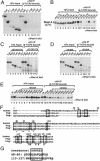
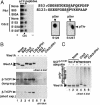
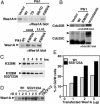
Wee1, the Cdc2 inhibitory kinase, needs to be down-regulated at the onset of mitosis to ensure rapid activation of Cdc2. Previously, we have shown that human somatic Wee1 (Wee1A) is down-regulated both by protein phosphorylation and degradation, but the underlying mechanisms had not been elucidated. In the present study, we have identified the beta-transducin repeat-containing protein 1/2 (beta-TrCP1/2) F-box protein-containing SKP1/Cul1/F-box protein (SCF) complex (SCF(beta-TrCP1/2)) as an E3 ubiquitin ligase for Wee1A ubiquitination. Although Wee1A lacks a consensus DS(p)GXXS(p) phospho-dependent binding motif for beta-TrCP, recognition of Wee1A by beta-TrCP depended on phosphorylation, and two serine residues in Wee1A, S53 and S123, were found to be the most important phosphorylation sites for beta-TrCP recognition. We have found also that the major M-phase kinases polo-like kinase 1 (Plk1) and Cdc2 are responsible for the phosphorylation of S53 and S123, respectively, and that in each case phosphorylation generates an unconventional phospho-degron (signal for degradation) that can be recognized by beta-TrCP. Phosphorylation of Wee1A by these kinases cooperatively stimulated the recognition and ubiquitination of Wee1A by SCF(beta-TrCP1/2) in vitro. Mutation of these residues or depletion of beta-TrCP by small-interfering RNA treatment increased the stability of Wee1A in HeLa cells. Moreover, our analysis indicates that beta-TrCP-dependent degradation of Wee1A is important for the normal onset of M-phase in vivo. These results also establish the existence of a feedback loop between Cdc2 and Wee1A in somatic cells that depends on ubiquitination and protein degradation and ensures the rapid activation of Cdc2 when cells are ready to divide.
Figures
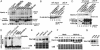
Similar articles
-
Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways.Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11663-8. doi: 10.1073/pnas.0500410102. Epub 2005 Aug 5. Proc Natl Acad Sci U S A. 2005. PMID: 16085715 Free PMC article.
-
Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome.Proc Natl Acad Sci U S A. 2004 May 25;101(21):7937-42. doi: 10.1073/pnas.0402442101. Epub 2004 May 17. Proc Natl Acad Sci U S A. 2004. PMID: 15148369 Free PMC article.
-
The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro.Genes Dev. 1999 Feb 1;13(3):270-83. doi: 10.1101/gad.13.3.270. Genes Dev. 1999. PMID: 9990852 Free PMC article.
-
The characteristics and roles of β-TrCP1/2 in carcinogenesis.FEBS J. 2021 Jun;288(11):3351-3374. doi: 10.1111/febs.15585. Epub 2020 Oct 23. FEBS J. 2021. PMID: 33021036 Review.
-
DEPTOR ubiquitination and destruction by SCF(β-TrCP).Am J Physiol Endocrinol Metab. 2012 Jul 15;303(2):E163-9. doi: 10.1152/ajpendo.00105.2012. Epub 2012 Mar 27. Am J Physiol Endocrinol Metab. 2012. PMID: 22454292 Free PMC article. Review.
Cited by
-
Revealing β-TrCP activity dynamics in live cells with a genetically encoded biosensor.Nat Commun. 2022 Oct 26;13(1):6364. doi: 10.1038/s41467-022-33762-3. Nat Commun. 2022. PMID: 36289220 Free PMC article.
-
The Apparent Requirement for Protein Synthesis during G2 Phase Is due to Checkpoint Activation.Cell Rep. 2020 Jul 14;32(2):107901. doi: 10.1016/j.celrep.2020.107901. Cell Rep. 2020. PMID: 32668239 Free PMC article.
-
Clinical significance of FBXW7 loss of function in human cancers.Mol Cancer. 2022 Mar 26;21(1):87. doi: 10.1186/s12943-022-01548-2. Mol Cancer. 2022. PMID: 35346215 Free PMC article. Review.
-
Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast.EMBO J. 2005 Jun 15;24(12):2194-204. doi: 10.1038/sj.emboj.7600683. Epub 2005 May 26. EMBO J. 2005. PMID: 15920482 Free PMC article.
-
Suppression of the Schizosaccharomyces pombe cut12.1 cell-cycle defect by mutations in cdc25 and genes involved in transcriptional and translational control.Genetics. 2007 May;176(1):73-83. doi: 10.1534/genetics.107.072090. Epub 2007 Apr 3. Genetics. 2007. PMID: 17409062 Free PMC article.
References
-
- Booher, R. N., Holman, P. S. & Fattaey, A. (1997) J. Biol. Chem. 272, 22300–22306. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

