Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac
- PMID: 15067024
- PMCID: PMC2172091
- DOI: 10.1083/jcb.200307109
Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac
Abstract
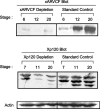
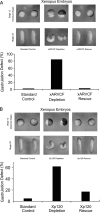
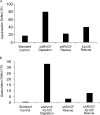
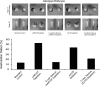
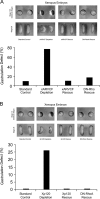
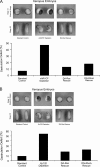
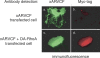
Using an animal model system and depletion-rescue strategies, we have addressed the requirement and functions of armadillo repeat gene deleted in velo-cardio-facial syndrome (ARVCF) and p120 catenins in early vertebrate embryogenesis. We find that xARVCF and Xp120 are essential to development given that depletion of either results in disrupted gastrulation and axial elongation, which are specific phenotypes based on self-rescue analysis and further criteria. Exogenous xARVCF or Xp120 cross-rescued depletion of the other, and each depletion was additionally rescued with (carefully titrated) dominant-negative RhoA or dominant-active Rac. Although xARVCF or Xp120 depletion did not appear to reduce the adhesive function of C-cadherin in standard cell reaggregation and additional assays, C-cadherin levels were somewhat reduced after xARVCF or Xp120 depletion, and rescue analysis using partial or full-length C-cadherin constructs suggested contributory effects on altered adhesion and signaling functions. This work indicates the required functions of both p120 and ARVCF in vertebrate embryogenesis and their shared functional interplay with RhoA, Rac, and cadherin in a developmental context.
Figures


Similar articles
-
Xenopus delta-catenin is essential in early embryogenesis and is functionally linked to cadherins and small GTPases.J Cell Sci. 2009 Nov 15;122(Pt 22):4049-61. doi: 10.1242/jcs.031948. Epub 2009 Oct 20. J Cell Sci. 2009. PMID: 19843587 Free PMC article.
-
Xenopus Kazrin interacts with ARVCF-catenin, spectrin and p190B RhoGAP, and modulates RhoA activity and epithelial integrity.J Cell Sci. 2010 Dec 1;123(Pt 23):4128-44. doi: 10.1242/jcs.072041. Epub 2010 Nov 9. J Cell Sci. 2010. PMID: 21062899 Free PMC article.
-
Xarvcf, Xenopus member of the p120 catenin subfamily associating with cadherin juxtamembrane region.J Biol Chem. 2000 Sep 29;275(39):30124-31. doi: 10.1074/jbc.M003048200. J Biol Chem. 2000. PMID: 10899158
-
Beyond regulation of cell adhesion: local control of RhoA at the cleavage furrow by the p0071 catenin.Cell Cycle. 2007 Jan 15;6(2):122-7. doi: 10.4161/cc.6.2.3741. Epub 2007 Jan 19. Cell Cycle. 2007. PMID: 17264675 Review.
-
Developmental functions of the P120-catenin sub-family.Biochim Biophys Acta. 2007 Jan;1773(1):17-33. doi: 10.1016/j.bbamcr.2006.06.009. Epub 2006 Jul 1. Biochim Biophys Acta. 2007. PMID: 16942809 Review.
Cited by
-
Adherens junctions in myelinating Schwann cells stabilize Schmidt-Lanterman incisures via recruitment of p120 catenin to E-cadherin.J Neurosci. 2005 Mar 30;25(13):3259-69. doi: 10.1523/JNEUROSCI.5168-04.2005. J Neurosci. 2005. PMID: 15800180 Free PMC article.
-
Intracellular Signals Activated by Canonical Wnt Ligands Independent of GSK3 Inhibition and β-Catenin Stabilization.Cells. 2019 Sep 25;8(10):1148. doi: 10.3390/cells8101148. Cells. 2019. PMID: 31557964 Free PMC article. Review.
-
p120-Catenin is required for mouse vascular development.Circ Res. 2010 Mar 19;106(5):941-51. doi: 10.1161/CIRCRESAHA.109.207753. Epub 2010 Jan 28. Circ Res. 2010. PMID: 20110533 Free PMC article.
-
p120-catenin regulates REST and CoREST, and modulates mouse embryonic stem cell differentiation.J Cell Sci. 2014 Sep 15;127(Pt 18):4037-51. doi: 10.1242/jcs.151944. Epub 2014 Jul 29. J Cell Sci. 2014. PMID: 25074806 Free PMC article.
-
Phosphorylation and isoform use in p120-catenin during development and tumorigenesis.Biochim Biophys Acta. 2016 Jan;1863(1):102-14. doi: 10.1016/j.bbamcr.2015.10.008. Epub 2015 Oct 23. Biochim Biophys Acta. 2016. PMID: 26477567 Free PMC article. Review.
References
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2000. The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113:1319–1334. - PubMed
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13:604–610. - PubMed
-
- Anastasiadis, P.Z., S.Y. Moon, M.A. Thoreson, D.J. Mariner, H.C. Crawford, Y. Zheng, and A.B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637–644. - PubMed
-
- Angres, B., A.H.J. Müller, J. Kellermann, and P. Hausen. 1991. Differential expression of two cadherins in Xenopus laevis. Development. 111:829–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

