Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer
- PMID: 15065984
- PMCID: PMC1133789
- DOI: 10.1042/BJ20040321
Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer
Abstract
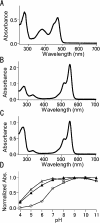
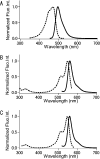
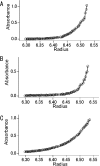
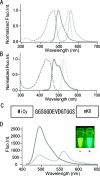
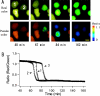
GFP (green fluorescent protein)-based FRET (fluorescence resonance energy transfer) technology has facilitated the exploration of the spatio-temporal patterns of cellular signalling. While most studies have used cyan- and yellow-emitting FPs (fluorescent proteins) as FRET donors and acceptors respectively, this pair of proteins suffers from problems of pH-sensitivity and bleeding between channels. In the present paper, we demonstrate the use of an alternative additional donor/acceptor pair. We have cloned two genes encoding FPs from stony corals. We isolated a cyan-emitting FP from Acropara sp., whose tentacles exhibit cyan coloration. Similar to GFP from Renilla reniformis, the cyan FP forms a tight dimeric complex. We also discovered an orange-emitting FP from Fungia concinna. As the orange FP exists in a complex oligomeric structure, we converted this protein into a monomeric form through the introduction of three amino acid substitutions, recently reported to be effective for converting DsRed into a monomer (Clontech). We used the cyan FP and monomeric orange FP as a donor/acceptor pair to monitor the activity of caspase 3 during apoptosis. Due to the close spectral overlap of the donor emission and acceptor absorption (a large Förster distance), substantial pH-resistance of the donor fluorescence quantum yield and the acceptor absorbance, as well as good separation of the donor and acceptor signals, the new pair can be used for more effective quantitative FRET imaging.
Figures

Similar articles
-
Measuring caspase activity by Förster resonance energy transfer.Cold Spring Harb Protoc. 2015 Jan 5;2015(1):pdb.prot082560. doi: 10.1101/pdb.prot082560. Cold Spring Harb Protoc. 2015. PMID: 25561624
-
Improving the spectral analysis of Fluorescence Resonance Energy Transfer in live cells: application to interferon receptors and Janus kinases.Cytokine. 2013 Oct;64(1):272-85. doi: 10.1016/j.cyto.2013.05.026. Epub 2013 Jun 21. Cytokine. 2013. PMID: 23796694 Free PMC article.
-
Flow cytometric measurement of fluorescence (Förster) resonance energy transfer from cyan fluorescent protein to yellow fluorescent protein using single-laser excitation at 458 nm.Cytometry A. 2003 May;53(1):39-54. doi: 10.1002/cyto.a.10037. Cytometry A. 2003. PMID: 12701131
-
The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins.Nat Biotechnol. 2004 Mar;22(3):289-96. doi: 10.1038/nbt943. Nat Biotechnol. 2004. PMID: 14990950 Review.
-
A Guide to Fluorescent Protein FRET Pairs.Sensors (Basel). 2016 Sep 14;16(9):1488. doi: 10.3390/s16091488. Sensors (Basel). 2016. PMID: 27649177 Free PMC article. Review.
Cited by
-
A monomeric red fluorescent protein with low cytotoxicity.Nat Commun. 2012;3:1204. doi: 10.1038/ncomms2208. Nat Commun. 2012. PMID: 23149748
-
New alternately colored FRET sensors for simultaneous monitoring of Zn²⁺ in multiple cellular locations.PLoS One. 2012;7(11):e49371. doi: 10.1371/journal.pone.0049371. Epub 2012 Nov 16. PLoS One. 2012. PMID: 23173058 Free PMC article.
-
Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology.J Physiol. 2007 Jan 1;578(Pt 1):55-67. doi: 10.1113/jphysiol.2006.120212. Epub 2006 Oct 12. J Physiol. 2007. PMID: 17038427 Free PMC article. Review.
-
Shining light on signaling and metabolic networks by genetically encoded biosensors.Curr Opin Plant Biol. 2005 Dec;8(6):574-81. doi: 10.1016/j.pbi.2005.09.015. Epub 2005 Sep 26. Curr Opin Plant Biol. 2005. PMID: 16188489 Free PMC article. Review.
-
Engineering and characterizing monomeric fluorescent proteins for live-cell imaging applications.Nat Protoc. 2014 Apr;9(4):910-28. doi: 10.1038/nprot.2014.054. Epub 2014 Mar 20. Nat Protoc. 2014. PMID: 24651502
References
-
- Tsien R. Y. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. - PubMed
-
- Matz M. V., Fradokov A. F., Labas Y. A., Savitsky A. P., Zaraisky A. G., Markelov M. L., Lukyanov S. A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999;17:969–973. - PubMed
-
- Miyawaki A. Green fluorescent protein-like proteins in reef Anthozoa animals. Cell Struct. Funct. 2002;27:343–347. - PubMed
-
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

