Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants
- PMID: 15056757
- PMCID: PMC395908
- DOI: 10.1073/pnas.0305143101
Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants
Abstract
More than 90 point mutations in human CuZn superoxide dismutase lead to the development of familial amyotrophic lateral sclerosis, known also as motor neuron disease. A growing body of evidence suggests that a subset of mutations located close to the dimeric interface can lead to a major destabilization of the mutant enzymes. We have determined the crystal structures of the Ala4Val (A4V) and Ile113Thr (I113T) mutants to 1.9 and 1.6 A, respectively. In the A4V structure, small changes at the dimer interface result in a substantial reorientation of the two monomers. This effect is also seen in the case of the I113T crystal structure, but to a smaller extent. X-ray solution scattering data show that in the solution state, both of the mutants undergo a more pronounced conformational change compared with wild-type superoxide dismutase (SOD) than that observed in the A4V crystal structure. Shape reconstructions from the x-ray scattering data illustrate the nature of this destabilization. Comparison of these scattering data with those for bovine CuZn SOD measured at different temperatures shows that an analogous change in the scattering profile occurs for the bovine enzyme in the temperature range of 70-80 degrees C. These results demonstrate that the A4V and I113T mutants are substantially destabilized in comparison with wild-type SOD1, and it is possible that the pathogenic properties of this subset of familial amyotrophic lateral sclerosis mutants are at least in part due to this destabilization.
Figures
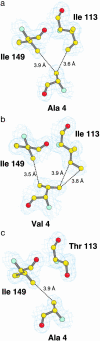
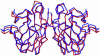
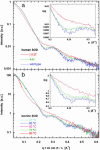
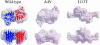
Comment in
-
A possible therapeutic target for Lou Gehrig's disease.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5701-2. doi: 10.1073/pnas.0401934101. Epub 2004 Apr 12. Proc Natl Acad Sci U S A. 2004. PMID: 15079068 Free PMC article. No abstract available.
Similar articles
-
An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial amytrophic lateral sclerosis.Biochemistry. 2004 May 4;43(17):4899-905. doi: 10.1021/bi030246r. Biochemistry. 2004. PMID: 15109247
-
Common dynamical signatures of familial amyotrophic lateral sclerosis-associated structurally diverse Cu, Zn superoxide dismutase mutants.Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3147-52. doi: 10.1073/pnas.0511266103. Epub 2006 Feb 17. Proc Natl Acad Sci U S A. 2006. PMID: 16488975 Free PMC article.
-
Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study.Comput Biol Med. 2022 Dec;151(Pt B):106319. doi: 10.1016/j.compbiomed.2022.106319. Epub 2022 Nov 24. Comput Biol Med. 2022. PMID: 36446187
-
Mutant SOD1 instability: implications for toxicity in amyotrophic lateral sclerosis.Neurodegener Dis. 2005;2(3-4):115-27. doi: 10.1159/000089616. Neurodegener Dis. 2005. PMID: 16909016 Review.
-
[Amyotrophic lateral sclerosis and superoxide dismutase--a review].Ugeskr Laeger. 1997 Mar 10;159(11):1593-6. Ugeskr Laeger. 1997. PMID: 9092140 Review. Danish.
Cited by
-
Discovery of Novel Inhibitors against ALS-Related SOD1(A4V) Aggregation through the Screening of a Chemical Library Using Differential Scanning Fluorimetry (DSF).Pharmaceuticals (Basel). 2024 Sep 27;17(10):1286. doi: 10.3390/ph17101286. Pharmaceuticals (Basel). 2024. PMID: 39458929 Free PMC article.
-
Mutation/metal deficiency in the "electrostatic loop" enhanced aggregation process in apo/holo SOD1 variants: implications for ALS diseases.BMC Chem. 2024 Sep 19;18(1):177. doi: 10.1186/s13065-024-01289-x. BMC Chem. 2024. PMID: 39300574 Free PMC article.
-
The prion-like effect and prion-like protein targeting strategy in amyotrophic lateral sclerosis.Heliyon. 2024 Jul 22;10(15):e34963. doi: 10.1016/j.heliyon.2024.e34963. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170125 Free PMC article. Review.
-
Ebselen analogues delay disease onset and its course in fALS by on-target SOD-1 engagement.Sci Rep. 2024 May 27;14(1):12118. doi: 10.1038/s41598-024-62903-5. Sci Rep. 2024. PMID: 38802492 Free PMC article.
-
CATI: an efficient gene integration method for rodent and primate embryos by MMEJ suppression.Genome Biol. 2023 Jun 23;24(1):146. doi: 10.1186/s13059-023-02987-w. Genome Biol. 2023. PMID: 37353834 Free PMC article.
References
-
- Brown, R. H., Jr. (1995) Cell 80, 687–692. - PubMed
-
- Maier, C. M. & Chan, P. K. (2002) Neuroscientist 8, 323–334. - PubMed
-
- Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., et al. (1993) Nature 362, 59–56. - PubMed
-
- Gaudette, M., Hirano, M. & Siddique, T. (2000) Amyotroph. Lateral Scler. 1, 83–89. - PubMed
-
- Cleveland, D. W. & Rothstein, J. D. (2001) Nat. Rev. Neurosci. 11, 806–819. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

