Brain-specific deletion of neuropathy target esterase/swisscheese results in neurodegeneration
- PMID: 15051870
- PMCID: PMC387376
- DOI: 10.1073/pnas.0401030101
Brain-specific deletion of neuropathy target esterase/swisscheese results in neurodegeneration
Abstract
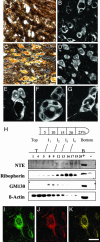
Neuropathy target esterase (NTE) is a neuronal membrane protein originally identified for its property to be modified by organo-phosphates (OPs), which in humans cause neuropathy characterized by axonal degeneration. Drosophila mutants for the homolog gene of NTE, swisscheese (sws), indicated a possible involvement of sws in the regulation of axon-glial cell interaction during glial wrapping. However, the role of NTE/sws in mammalian brain pathophysiology remains unknown. To investigate NTE function in vivo, we used the cre/loxP site-specific recombination strategy to generate mice with a specific deletion of NTE in neuronal tissues. Here we show that loss of NTE leads to prominent neuronal pathology in the hippocampus and thalamus and also defects in the cerebellum. Absence of NTE resulted in disruption of the endoplasmic reticulum, vacuolation of nerve cell bodies, and abnormal reticular aggregates. Thus, these results identify a physiological role for NTE in the nervous system and indicate that a loss-of-function mechanism may contribute to neurodegenerative diseases characterized by vacuolation and neuronal loss.
Figures




Similar articles
-
Loss of Swiss cheese/neuropathy target esterase activity causes disruption of phosphatidylcholine homeostasis and neuronal and glial death in adult Drosophila.J Neurosci. 2005 Mar 16;25(11):2865-73. doi: 10.1523/JNEUROSCI.5097-04.2005. J Neurosci. 2005. PMID: 15772346 Free PMC article.
-
Neuropathy target esterase: an essential enzyme for neural development and axonal maintenance.Int J Biochem Cell Biol. 2010 May;42(5):573-5. doi: 10.1016/j.biocel.2009.12.007. Epub 2009 Dec 16. Int J Biochem Cell Biol. 2010. PMID: 20006730 Review.
-
Glial expression of Swiss cheese (SWS), the Drosophila orthologue of neuropathy target esterase (NTE), is required for neuronal ensheathment and function.Dis Model Mech. 2016 Mar;9(3):283-94. doi: 10.1242/dmm.022236. Epub 2015 Dec 3. Dis Model Mech. 2016. PMID: 26634819 Free PMC article.
-
Unraveling the link between neuropathy target esterase NTE/SWS, lysosomal storage diseases, inflammation, abnormal fatty acid metabolism, and leaky brain barrier.Elife. 2024 Apr 25;13:e98020. doi: 10.7554/eLife.98020. Elife. 2024. PMID: 38660940 Free PMC article.
-
Axonal degeneration and neuropathy target esterase.Arh Hig Rada Toksikol. 2007 Sep;58(3):355-8. doi: 10.2478/v10004-007-0029-z. Arh Hig Rada Toksikol. 2007. PMID: 18050888 Review.
Cited by
-
Probing mechanisms that underlie human neurodegenerative diseases in Drosophila.Annu Rev Genet. 2012;46:371-96. doi: 10.1146/annurev-genet-110711-155456. Epub 2012 Sep 4. Annu Rev Genet. 2012. PMID: 22974305 Free PMC article. Review.
-
Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness.Nat Commun. 2015 Jan 9;6:5614. doi: 10.1038/ncomms6614. Nat Commun. 2015. PMID: 25574898 Free PMC article.
-
The Catalytic Domain of Neuropathy Target Esterase Influences Lipid Droplet Biogenesis and Lipid Metabolism in Human Neuroblastoma Cells.Metabolites. 2022 Jul 12;12(7):637. doi: 10.3390/metabo12070637. Metabolites. 2022. PMID: 35888761 Free PMC article.
-
ER responses play a key role in Swiss-Cheese/Neuropathy Target Esterase-associated neurodegeneration.Neurobiol Dis. 2019 Oct;130:104520. doi: 10.1016/j.nbd.2019.104520. Epub 2019 Jun 22. Neurobiol Dis. 2019. PMID: 31233884 Free PMC article.
-
Interaction of the Lysophospholipase PNPLA7 with Lipid Droplets through the Catalytic Region.Mol Cells. 2020 Mar 31;43(3):286-297. doi: 10.14348/molcells.2020.2283. Mol Cells. 2020. PMID: 32208367 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

