The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis
- PMID: 15047841
- PMCID: PMC374293
- DOI: 10.1128/jvi.78.8.4278-4288.2004
The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis
Abstract
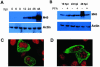
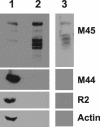
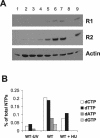
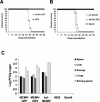
Ribonucleotide reductase (RNR) is the key enzyme in the biosynthesis of deoxyribonucleotides. Alpha- and gammaherpesviruses express a functional enzyme, since they code for both the R1 and the R2 subunits. By contrast, betaherpesviruses contain an open reading frame (ORF) with homology to R1, but an ORF for R2 is absent, suggesting that they do not express a functional RNR. The M45 protein of murine cytomegalovirus (MCMV) exhibits the sequence features of a class Ia RNR R1 subunit but lacks certain amino acid residues believed to be critical for enzymatic function. It starts to be expressed independently upon the onset of viral DNA synthesis at 12 h after infection and accumulates at later times in the cytoplasm of the infected cells. Moreover, it is associated with the virion particle. To investigate direct involvement of the virally encoded R1 subunit in ribonucleotide reduction, recombinant M45 was tested in enzyme activity assays together with cellular R1 and R2. The results indicate that M45 neither is a functional equivalent of an R1 subunit nor affects the activity or the allosteric control of the mouse enzyme. To replicate in quiescent cells, MCMV induces the expression and activity of the cellular RNR. Mutant viruses in which the M45 gene has been inactivated are avirulent in immunodeficient SCID mice and fail to replicate in their target organs. These results suggest that M45 has evolved a new function that is indispensable for virus replication and pathogenesis in vivo.
Figures


Similar articles
-
Expression of an altered ribonucleotide reductase activity associated with the replication of murine cytomegalovirus in quiescent fibroblasts.J Virol. 2000 Dec;74(24):11557-65. doi: 10.1128/jvi.74.24.11557-11565.2000. J Virol. 2000. PMID: 11090153 Free PMC article.
-
A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism.Science. 2001 Jan 12;291(5502):303-5. doi: 10.1126/science.291.5502.303. Science. 2001. PMID: 11209080
-
Cooperative inhibition of RIP1-mediated NF-κB signaling by cytomegalovirus-encoded deubiquitinase and inactive homolog of cellular ribonucleotide reductase large subunit.PLoS Pathog. 2017 Jun 1;13(6):e1006423. doi: 10.1371/journal.ppat.1006423. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28570668 Free PMC article.
-
Tinkering with a viral ribonucleotide reductase.Trends Biochem Sci. 2009 Jan;34(1):25-32. doi: 10.1016/j.tibs.2008.09.008. Epub 2008 Nov 5. Trends Biochem Sci. 2009. PMID: 18990579 Review.
-
Genetic analyses of gene function and pathogenesis of murine cytomegalovirus by transposon-mediated mutagenesis.J Clin Virol. 2002 Aug;25 Suppl 2:S111-22. doi: 10.1016/s1386-6532(02)00096-3. J Clin Virol. 2002. PMID: 12361762 Review.
Cited by
-
A temporal gate for viral enhancers to co-opt Toll-like-receptor transcriptional activation pathways upon acute infection.PLoS Pathog. 2015 Apr 9;11(4):e1004737. doi: 10.1371/journal.ppat.1004737. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25856589 Free PMC article.
-
Herpes simplex virus suppresses necroptosis in human cells.Cell Host Microbe. 2015 Feb 11;17(2):243-51. doi: 10.1016/j.chom.2015.01.003. Cell Host Microbe. 2015. PMID: 25674983 Free PMC article.
-
Block of death-receptor apoptosis protects mouse cytomegalovirus from macrophages and is a determinant of virulence in immunodeficient hosts.PLoS Pathog. 2012;8(12):e1003062. doi: 10.1371/journal.ppat.1003062. Epub 2012 Dec 13. PLoS Pathog. 2012. PMID: 23271968 Free PMC article.
-
A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16.Mol Cell Biol. 2005 Nov;25(21):9175-88. doi: 10.1128/MCB.25.21.9175-9188.2005. Mol Cell Biol. 2005. PMID: 16227571 Free PMC article.
-
Mouse newborn cells allow highly productive mouse cytomegalovirus replication, constituting a novel convenient primary cell culture system.PLoS One. 2017 Mar 24;12(3):e0174695. doi: 10.1371/journal.pone.0174695. eCollection 2017. PLoS One. 2017. PMID: 28339479 Free PMC article.
References
-
- Averett, D. R., C. Lubbers, G. B. Elion, and T. Spector. 1983. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J. Biol. Chem. 258:9831-9838. - PubMed
-
- Bjorklund, S., S. Skog, B. Tribukait, and L. Thelander. 1990. S-phase-specific expression of mammalian ribonucleotide reductase R1 and R2 subunit mRNAs. Biochemistry 29:5452-5458. - PubMed
-
- Brautigam, A. R., F. J. Dutko, L. B. Olding, and M. B. Oldstone. 1979. Pathogenesis of murine cytomegalovirus infection: the macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J. Gen. Virol. 44:349-359. - PubMed
-
- Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

