Detection of SARS coronavirus in patients with suspected SARS
- PMID: 15030700
- PMCID: PMC3322905
- DOI: 10.3201/eid1002.030610
Detection of SARS coronavirus in patients with suspected SARS
Abstract
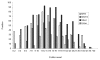
Cases of severe acute respiratory syndrome (SARS) were investigated for SARS coronavirus (SARS-CoV) through RNA tests, serologic response, and viral culture. Of 537 specimens from patients in whom SARS was clinically diagnosed, 332 (60%) had SARS-CoV RNA in one or more clinical specimens, compared with 1 (0.3%) of 332 samples from controls. Of 417 patients with clinical SARS from whom paired serum samples were available, 92% had an antibody response. Rates of viral RNA positivity increased progressively and peaked at day 11 after onset of illness. Although viral RNA remained detectable in respiratory secretions and stool and urine specimens for >30 days in some patients, virus could not be cultured after week 3 of illness. Nasopharyngeal aspirates, throat swabs, or sputum samples were the most useful clinical specimens in the first 5 days of illness, but later in the illness viral RNA could be detected more readily in stool specimens.
Figures
Similar articles
-
Evaluation of advanced reverse transcription-PCR assays and an alternative PCR target region for detection of severe acute respiratory syndrome-associated coronavirus.J Clin Microbiol. 2004 May;42(5):2043-7. doi: 10.1128/JCM.42.5.2043-2047.2004. J Clin Microbiol. 2004. PMID: 15131168 Free PMC article.
-
Laboratory diagnosis of SARS.Emerg Infect Dis. 2004 May;10(5):825-31. doi: 10.3201/eid1005.030682. Emerg Infect Dis. 2004. PMID: 15200815 Free PMC article.
-
Multicenter comparison of nucleic acid extraction methods for detection of severe acute respiratory syndrome coronavirus RNA in stool specimens.J Clin Microbiol. 2006 Aug;44(8):2681-8. doi: 10.1128/JCM.02460-05. J Clin Microbiol. 2006. PMID: 16891478 Free PMC article.
-
[Detection of SARS coronavirus RNA].Nihon Rinsho. 2005 Dec;63 Suppl 12:366-71. Nihon Rinsho. 2005. PMID: 16416820 Review. Japanese. No abstract available.
-
Molecular diagnosis of severe acute respiratory syndrome: the state of the art.J Mol Diagn. 2005 Nov;7(5):551-9. doi: 10.1016/S1525-1578(10)60587-9. J Mol Diagn. 2005. PMID: 16258152 Free PMC article. Review.
Cited by
-
Risk Factors Associated with Prolonged Nasopharyngeal Carriage of SARS-CoV-2 and Length of Stay among Patients Admitted to a COVID-19 Referral Center in Manila, Philippines.Acta Med Philipp. 2023 Dec 18;57(12):66-72. doi: 10.47895/amp.vi0.5764. eCollection 2023. Acta Med Philipp. 2023. PMID: 39429759 Free PMC article.
-
Detection of Alpha- and Betacoronaviruses in Small Mammals in Western Yunnan Province, China.Viruses. 2023 Sep 20;15(9):1965. doi: 10.3390/v15091965. Viruses. 2023. PMID: 37766371 Free PMC article.
-
Epidemiological features and consequences of COVID-19 in patients with and without gastrointestinal symptoms in southwestern Iran. A retrospective observational study.Health Sci Rep. 2023 Sep 18;6(9):e1499. doi: 10.1002/hsr2.1499. eCollection 2023 Sep. Health Sci Rep. 2023. PMID: 37732104 Free PMC article.
-
Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies.Arch Microbiol Immunol. 2023;7(2):36-61. Epub 2023 May 9. Arch Microbiol Immunol. 2023. PMID: 37388279 Free PMC article.
-
Pleiotropic Functions of Nitric Oxide Produced by Ascorbate for the Prevention and Mitigation of COVID-19: A Revaluation of Pauling's Vitamin C Therapy.Microorganisms. 2023 Feb 3;11(2):397. doi: 10.3390/microorganisms11020397. Microorganisms. 2023. PMID: 36838362 Free PMC article. Review.
References
-
- World Health Organization. Severe acute respiratory syndrome (SARS): over 100 days into the outbreak. Wkly Epidemiol Rec. 2003;78:217–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
