In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination
- PMID: 15024047
- PMCID: PMC2212731
- DOI: 10.1084/jem.20032220
In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination
Abstract
The prevention and treatment of prevalent infectious diseases and tumors should benefit from improvements in the induction of antigen-specific T cell immunity. To assess the potential of antigen targeting to dendritic cells to improve immunity, we incorporated ovalbumin protein into a monoclonal antibody to the DEC-205 receptor, an endocytic receptor that is abundant on these cells in lymphoid tissues. Simultaneously, we injected agonistic alpha-CD40 antibody to mature the dendritic cells. We found that a single low dose of antibody-conjugated ovalbumin initiated immunity from the naive CD4+ and CD8+ T cell repertoire. Unexpectedly, the alphaDEC-205 antigen conjugates, given s.c., targeted to dendritic cells systemically and for long periods, and ovalbumin peptide was presented on MHC class I for 2 weeks. This was associated with stronger CD8+ T cell-mediated immunity relative to other forms of antigen delivery, even when the latter was given at a thousand times higher doses. In parallel, the mice showed enhanced resistance to an established rapidly growing tumor and to viral infection at a mucosal site. By better harnessing the immunizing functions of maturing dendritic cells, antibody-mediated antigen targeting via the DEC-205 receptor increases the efficiency of vaccination for T cell immunity, including systemic and mucosal resistance in disease models.
Figures




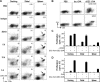
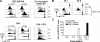
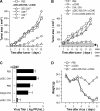
Similar articles
-
Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance.J Exp Med. 2002 Dec 16;196(12):1627-38. doi: 10.1084/jem.20021598. J Exp Med. 2002. PMID: 12486105 Free PMC article.
-
Chemical Conjugation of a Purified DEC-205-Directed Antibody with Full-Length Protein for Targeting Mouse Dendritic Cells In Vitro and In Vivo.J Vis Exp. 2021 Feb 5;(168). doi: 10.3791/62018. J Vis Exp. 2021. PMID: 33616089
-
Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic toll-like receptor ligands.BMC Immunol. 2009 Aug 3;10:43. doi: 10.1186/1471-2172-10-43. BMC Immunol. 2009. PMID: 19650904 Free PMC article.
-
Targeted antigen delivery to DEC-205⁺ dendritic cells for tolerogenic vaccination.Rev Diabet Stud. 2012 Winter;9(4):305-18. doi: 10.1900/RDS.2012.9.305. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804268 Free PMC article. Review.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
Cited by
-
STAT3 signaling modulates the immune response induced after antigen targeting to conventional type 1 dendritic cells through the DEC205 receptor.Front Immunol. 2022 Oct 18;13:1006996. doi: 10.3389/fimmu.2022.1006996. eCollection 2022. Front Immunol. 2022. PMID: 36330518 Free PMC article.
-
Resident memory T cells form during persistent antigen exposure leading to allograft rejection.Sci Immunol. 2021 Mar 19;6(57):eabc8122. doi: 10.1126/sciimmunol.abc8122. Sci Immunol. 2021. PMID: 33741656 Free PMC article.
-
IL-12p40/IL-10 Producing preCD8α/Clec9A+ Dendritic Cells Are Induced in Neonates upon Listeria monocytogenes Infection.PLoS Pathog. 2016 Apr 13;12(4):e1005561. doi: 10.1371/journal.ppat.1005561. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27074026 Free PMC article.
-
Generation of hypoallergenic neoglycoconjugates for dendritic cell targeted vaccination: a novel tool for specific immunotherapy.J Control Release. 2013 Jan 28;165(2):101-9. doi: 10.1016/j.jconrel.2012.11.002. Epub 2012 Nov 10. J Control Release. 2013. PMID: 23147517 Free PMC article.
-
Induction of antigen-specific tolerance by nanobody-antigen adducts that target class-II major histocompatibility complexes.Nat Biomed Eng. 2021 Nov;5(11):1389-1401. doi: 10.1038/s41551-021-00738-5. Epub 2021 Jun 14. Nat Biomed Eng. 2021. PMID: 34127819
References
-
- Seder, R.A., and J.R. Mascola. 2003. Basic Immunology of Vaccine Development. Academic Press, Boston. 51–72.
-
- McMichael, A.J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874–880. - PubMed
-
- Reed, S.G., and A. Campos-Neto. 2003. Vaccines for parasitic and bacterial diseases. Curr. Opin. Immunol. 15:456–460. - PubMed
-
- Finn, O.J. 2003. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 3:630–641. - PubMed
-
- Lu, W., X. Wu, Y. Lu, W. Guo, and J.M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9:27–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

