Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-kappaB/IkappaB pathway
- PMID: 15016316
- PMCID: PMC394342
- DOI: 10.1186/1476-4598-3-7
Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-kappaB/IkappaB pathway
Abstract
Background: Cyclooxygenase-2 (COX-2) has been shown to be highly expressed in a broad series of primary endometrial tumors and its expression may be closely associated with parameters of tumor aggressiveness. In human endometrial cancer, tumor suppressor phosphatase tensin homologue (PTEN) is frequently mutated. In the presence of a mutated PTEN protein, Akt phosphorylation levels increase leading to the activation of this survival pathway. The nuclear transcription factor kappaB (NF-kappaB) is a well establish regulator of genes encoding cytokines, cytokine receptors, and cell adhesion molecules that drive immune and inflammatory responses. More recently, NF-kappaB activation has been connected with multiple aspects of oncogenesis, including the control of apoptosis, cell cycle, differentiation, and cell migration. It is known that Akt may act through NF-kappaB pathway and that COX-2 gene has been shown to be regulated at the promoter level by NF-kappaB. Recently, we showed that Akt regulates COX-2 gene and protein expressions in phospho-Akt expressing endometrial cancer cells. The present study was undertaken to determine the involvement of NF-kappaB pathway and IkappaB (an inhibitor of NF-kappaB) in the regulation of COX-2 expression and to determine more precisely the downstream targets of Akt involved in this process.
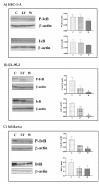
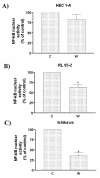
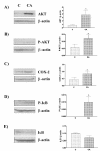
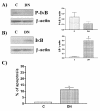
Results: Three different human endometrial cancer cell lines known to have wild type PTEN (HEC 1-A) or a mutated inactive PTEN protein (RL 95-2 and Ishikawa) were used for these studies. Expression IkappaB and Phospho-IkappaB were evaluated by Western analysis. The presence of IkappaB phosphorylation was found in all cell lines studied. There was no difference between cell lines in term of NF-kappaB abundance. Inhibition of PI 3-K with Wortmannin and LY294002 blocked IkappaB phosphorylation, reduced NF-kappaB nuclear activity, reduced COX-2 expression and induced apoptosis. Transfection studies with a dominant negative Akt vector blocked IkappaB phosphorylation and reduced COX-2 expression. On the opposite, constitutively active Akt transfections resulted in the induction of IkappaB phosphorylation and up-regulation of COX-2.
Conclusion: These results demonstrate that Akt signals through NF-kappaB/IkappaB pathway to induce COX-2 expression in mutated PTEN endometrial cancer cells.
Figures

Similar articles
-
Akt regulates COX-2 mRNA and protein expression in mutated-PTEN human endometrial cancer cells.Int J Oncol. 2004 May;24(5):1311-24. Int J Oncol. 2004. PMID: 15067356
-
The involvement of nuclear factor-kappa B in cyclooxygenase-2 overexpression in murine colon cancer cells transduced with herpes simplex virus thymidine kinase gene.Cancer Gene Ther. 2006 Dec;13(12):1093-104. doi: 10.1038/sj.cgt.7700983. Epub 2006 Jul 14. Cancer Gene Ther. 2006. PMID: 16841079
-
Akt activity in endometrial cancer cells: regulation of cell survival through cIAP-1.Int J Oncol. 2003 Sep;23(3):803-10. Int J Oncol. 2003. PMID: 12888921
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
It takes two to tango: IκBs, the multifunctional partners of NF-κB.Immunol Rev. 2012 Mar;246(1):59-76. doi: 10.1111/j.1600-065X.2012.01102.x. Immunol Rev. 2012. PMID: 22435547 Review.
Cited by
-
Hedysarum multijugum Maxim treats ulcerative colitis through the PI3K-AKT and TNF signaling pathway according to network pharmacology and molecular docking.Ann Transl Med. 2022 Oct;10(20):1132. doi: 10.21037/atm-22-4815. Ann Transl Med. 2022. PMID: 36388782 Free PMC article.
-
Mitochondria-targeted antioxidant SkQ1 inhibits leukotriene synthesis in human neutrophils.Front Pharmacol. 2022 Nov 24;13:1023517. doi: 10.3389/fphar.2022.1023517. eCollection 2022. Front Pharmacol. 2022. PMID: 36506526 Free PMC article.
-
Combined Ibuprofen-Nanoconjugate Micelles with E-Selectin for Effective Sunitinib Anticancer Therapy.Int J Nanomedicine. 2022 Dec 6;17:6031-6046. doi: 10.2147/IJN.S388234. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36510619 Free PMC article.
-
Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma.Biomolecules. 2019 Jun 27;9(7):253. doi: 10.3390/biom9070253. Biomolecules. 2019. PMID: 31252679 Free PMC article.
-
Prospects of NSAIDs administration as double-edged agents against endometrial cancer and pathological species of the uterine microbiome.Cancer Biol Ther. 2020 Jun 2;21(6):486-494. doi: 10.1080/15384047.2020.1736483. Epub 2020 Mar 15. Cancer Biol Ther. 2020. PMID: 32174282 Free PMC article. Review.
References
-
- Lenardo MJ, Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989;58:227–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

