Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients
- PMID: 15013977
- PMCID: PMC371224
- DOI: 10.1128/cdli.11.2.287-291.2004
Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients
Abstract
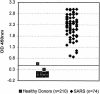
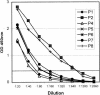
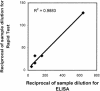
An enzyme-linked immunosorbent assay (ELISA) and a rapid immunochromatographic test for detection of immunoglobulin G (IgG) antibodies in severe acute respiratory syndrome (SARS) patients were developed by utilizing the well-characterized recombinant proteins Gst-N and Gst-U274. The ELISA detected IgG antibodies to SARS-CoV in all 74 convalescent-phase samples from SARS patients while weakly cross-reacting to only 1 of the 210 control sera from healthy donors. This finding thus led to a kit sensitivity, specificity, and accuracy of 100, 99.5, and 99.6%, respectively. The test thus provided a positive predictive value (PPV) of 98.7% and a negative predictive value (NPV) of 100%. In addition, the ELISA gave a positive delta of 5.4 and a negative delta of 3.6, indicating an excellent differentiation between positives and negatives. The same recombinant proteins were also applied to a newly developed platform for the development of a 15-min rapid test. The resulting rapid test has an excellent agreement of 99.6%, with a kappa value of 1.00, with the ELISA. Again, this rapid test was able to detect 100% of the samples tested (n = 42) while maintaining a specificity of 99.0% (n = 210). The PPV and NPV for the rapid test thus reached 95.3 and 100%, respectively.
Figures

Similar articles
-
Evaluation and validation of an enzyme-linked immunosorbent assay and an immunochromatographic test for serological diagnosis of severe acute respiratory syndrome.Clin Diagn Lab Immunol. 2004 Jul;11(4):699-703. doi: 10.1128/CDLI.11.4.699-703.2004. Clin Diagn Lab Immunol. 2004. PMID: 15242944 Free PMC article.
-
Recombinant nucleocapsid protein-based IgG enzyme-linked immunosorbent assay for the serological diagnosis of SARS.J Virol Methods. 2005 May;125(2):181-6. doi: 10.1016/j.jviromet.2005.01.028. J Virol Methods. 2005. PMID: 15794988 Free PMC article.
-
Development of immunoglobulin G enzyme-linked immunosorbent assay for the serodiagnosis of severe acute respiratory syndrome.J Biomed Sci. 2005;12(1):59-64. doi: 10.1007/s11373-004-8184-6. Epub 2005 Feb 4. J Biomed Sci. 2005. PMID: 15864739 Free PMC article.
-
Severe acute respiratory syndrome (SARS): development of diagnostics and antivirals.Ann N Y Acad Sci. 2006 May;1067(1):500-5. doi: 10.1196/annals.1354.072. Ann N Y Acad Sci. 2006. PMID: 16804033 Free PMC article. Review.
-
[Diagnostic tests: SARS-Corona virus].Nihon Rinsho. 2005 Jul;63 Suppl 7:343-5. Nihon Rinsho. 2005. PMID: 16111269 Review. Japanese. No abstract available.
Cited by
-
Recent advances in immunoassay technologies for the detection of human coronavirus infections.Front Cell Infect Microbiol. 2023 Jan 4;12:1040248. doi: 10.3389/fcimb.2022.1040248. eCollection 2022. Front Cell Infect Microbiol. 2023. PMID: 36683684 Free PMC article. Review.
-
Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review.Sensors (Basel). 2021 Dec 25;22(1):133. doi: 10.3390/s22010133. Sensors (Basel). 2021. PMID: 35009676 Free PMC article. Review.
-
Biochemical composition, transmission and diagnosis of SARS-CoV-2.Biosci Rep. 2021 Aug 27;41(8):BSR20211238. doi: 10.1042/BSR20211238. Biosci Rep. 2021. PMID: 34291285 Free PMC article. Review.
-
Improved diagnosis of SARS-CoV-2 by using nucleoprotein and spike protein fragment 2 in quantitative dual ELISA tests.Epidemiol Infect. 2021 Jun 8;149:e140. doi: 10.1017/S0950268821001308. Epidemiol Infect. 2021. PMID: 34099081 Free PMC article.
-
Development and Clinical Evaluation of an Immunochromatography-Based Rapid Antigen Test (GenBody™ COVAG025) for COVID-19 Diagnosis.Viruses. 2021 Apr 29;13(5):796. doi: 10.3390/v13050796. Viruses. 2021. PMID: 33946860 Free PMC article.
References
-
- Centers for Disease Control and Prevention. 2003. Updated interim surveillance case definition for severe acute respiratory syndrome (SARS)—United States, April 29, 2003. Morb. Mortal. Wkly. Rep. 52:1-3. - PubMed
-
- Crofts, N., W. Maskill, and D. Gust. 1988. Evaluation of enzyme-linked immunosorbent assays: a method of data analysis. J. Virol. Methods 22:51-59. - PubMed
-
- Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brot, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 384:1967-1976. - PubMed
-
- Feng, P., S. H. Chan, M. Y. R. Soo, D. X. Liu, M. Guan, E. R. Ren, and H. Z. Hu. 2001. Antibody response to Epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma. Cancer 92:1872-1880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

