Macrophage-derived tumor necrosis factor alpha, an early developmental signal for motoneuron death
- PMID: 14999074
- PMCID: PMC6730439
- DOI: 10.1523/JNEUROSCI.4464-03.2004
Macrophage-derived tumor necrosis factor alpha, an early developmental signal for motoneuron death
Abstract
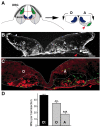
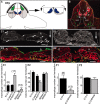
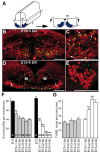
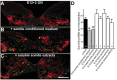
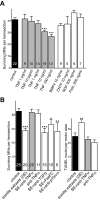
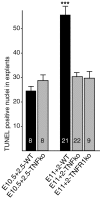
Mechanisms inducing neuronal death at defined times during embryogenesis remain enigmatic. We show in explants that a developmental switch occurs between embryonic day 12 (E12) and E13 in rats that is 72-48 hr before programmed cell death. Half the motoneurons isolated from peripheral tissues at E12 escape programmed cell death, whereas 90% of motoneurons isolated at E13 enter a death program. The surrounding somite commits E12 motoneurons to death. This effect requires macrophage cells, is mimicked by tumor necrosis factor alpha (TNFalpha), and is inhibited by anti-TNFalpha antibodies. In vivo, TNFalpha is detected within somite macrophages, and TNF receptor 1 (TNFR1) is detected within motoneurons precisely between E12 and E13. Although motoneuron cell death occurs normally in TNFalpha-/- mice, this process is significantly reduced in explants from TNFalpha-/- and TNFR1-/- mice. Thus, embryonic motoneurons acquire the competence to die, before the onset of programmed cell death, from extrinsic signals such as macrophage-derived TNFalpha
Figures
Similar articles
-
Necdin protects embryonic motoneurons from programmed cell death.PLoS One. 2011;6(9):e23764. doi: 10.1371/journal.pone.0023764. Epub 2011 Sep 2. PLoS One. 2011. PMID: 21912643 Free PMC article.
-
Cytotoxic potential of proinflammatory cytokines: combined deletion of TNF receptors TNFR1 and TNFR2 prevents motoneuron cell death after facial axotomy in adult mouse.Exp Neurol. 2002 Dec;178(2):186-93. doi: 10.1006/exnr.2002.8024. Exp Neurol. 2002. PMID: 12504878
-
Involvement of tumor necrosis factor-alpha in the pathogenesis of autoimmune orchitis in rats.Biol Reprod. 2003 Jun;68(6):2114-21. doi: 10.1095/biolreprod.102.011189. Epub 2002 Dec 27. Biol Reprod. 2003. PMID: 12606341
-
Shedding of TNFR1 in regenerative liver can be induced with TNF alpha and PMA.World J Gastroenterol. 2002 Dec;8(6):1129-33. doi: 10.3748/wjg.v8.i6.1129. World J Gastroenterol. 2002. PMID: 12439939 Free PMC article.
-
Tumor necrosis factor-alpha and neuronal development.Neuroscientist. 2005 Aug;11(4):277-81. doi: 10.1177/1073858404270905. Neuroscientist. 2005. PMID: 16061514 Review.
Cited by
-
The Contribution of Microglia to the Development and Maturation of the Visual System.Front Cell Neurosci. 2021 Apr 23;15:659843. doi: 10.3389/fncel.2021.659843. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33967697 Free PMC article.
-
The Role of Immune Factors in Shaping Fetal Neurodevelopment.Annu Rev Cell Dev Biol. 2020 Oct 6;36:441-468. doi: 10.1146/annurev-cellbio-021120-033518. Epub 2020 Jul 28. Annu Rev Cell Dev Biol. 2020. PMID: 32722920 Free PMC article. Review.
-
Myeloid masquerade: Microglial transcriptional signatures in retinal development and disease.Front Cell Neurosci. 2023 Jan 26;17:1106547. doi: 10.3389/fncel.2023.1106547. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36779012 Free PMC article. Review.
-
What the Spectrum of Microglial Functions Can Teach us About Fetal Alcohol Spectrum Disorder.Front Synaptic Neurosci. 2017 Jun 19;9:11. doi: 10.3389/fnsyn.2017.00011. eCollection 2017. Front Synaptic Neurosci. 2017. PMID: 28674490 Free PMC article. Review.
-
Astrocytes and microglia in the coordination of CNS development and homeostasis.J Neurochem. 2024 Oct;168(10):3599-3614. doi: 10.1111/jnc.16006. Epub 2023 Nov 20. J Neurochem. 2024. PMID: 37985374 Review.
References
-
- Altman J, Bayer SA (1984) The development of the rat spinal cord. Adv Anat Embryol Cell Biol 85: 1-164. - PubMed
-
- Barker V, Middleton G, Davey F, Davies AM (2001) TNFalpha contributes to the death of NGF-dependent neurons during development. Nat Neurosci 4: 1194-1198. - PubMed
-
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC (1994) Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett 172: 151-154. - PubMed
-
- Butler H, Juurlink BHJ (1987) An atlas for staging mammalian and chick embryos. Boca Raton, FL: CRC.
-
- Cuadros MA, Navascues J (1998) The origin and differentiation of microglial cells during development. Prog Neurobiol 56: 173-189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

