Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro
- PMID: 14990702
- PMCID: PMC353759
- DOI: 10.1128/jvi.78.6.2831-2840.2004
Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro
Abstract
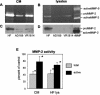
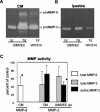
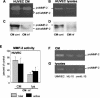
At the uterine-placental interface, fetal cytotrophoblasts invade the decidua, breach maternal blood vessels, and form heterotypic contacts with uterine microvascular endothelial cells. In early gestation, differentiating- invading cytotrophoblasts produce high levels of matrix metalloproteinase 9 (MMP-9), which degrades the extracellular matrix and increases the invasion depth. By midgestation, when invasion is complete, MMP levels are reduced. Cytotrophoblasts also produce human interleukin-10 (hIL-10), a pleiotropic cytokine that modulates immune responses, helping to protect the fetal hemiallograft from rejection. Human cytomegalovirus (CMV) is often detected at the uterine-placental interface. CMV infection impairs cytotrophoblast differentiation and invasion, altering the expression of the cell adhesion and immune molecules. Here we report that infection with a clinical CMV strain, VR1814, but not a laboratory strain, AD169, downregulates MMP activity in uterine microvascular endothelial cells and differentiating-invading cytotrophoblasts. Infected cytotrophoblasts expressed CMV IL-10 (cmvIL-10) mRNA and secreted the viral cytokine, which upregulated hIL-10. Functional analyses showed that cmvIL-10 treatment impaired migration in endothelial cell wounding assays and cytotrophoblast invasion of Matrigel in vitro. Comparable changes occurred in cells that were exposed to recombinant hIL-10 or cmvIL-10. Our results show that cmvIL-10 decreases MMP activity and dysregulates the cell-cell and/or cell-matrix interactions of infected cytotrophoblasts and endothelial cells. Reduced MMP activity early in placental development could impair cytotrophoblast remodeling of the uterine vasculature and eventually restrict fetal growth in affected pregnancies.
Figures



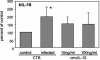
Similar articles
-
Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis.J Virol. 2000 Aug;74(15):6808-20. doi: 10.1128/jvi.74.15.6808-6820.2000. J Virol. 2000. PMID: 10888620 Free PMC article.
-
IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion.Dev Biol. 1999 Jan 1;205(1):194-204. doi: 10.1006/dbio.1998.9122. Dev Biol. 1999. PMID: 9882507
-
Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model.Am J Pathol. 2012 Nov;181(5):1540-59. doi: 10.1016/j.ajpath.2012.08.003. Epub 2012 Sep 7. Am J Pathol. 2012. PMID: 22959908 Free PMC article.
-
Why is placentation abnormal in preeclampsia?Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S115-22. doi: 10.1016/j.ajog.2015.08.042. Am J Obstet Gynecol. 2015. PMID: 26428489 Free PMC article. Review.
-
Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge.Curr Top Microbiol Immunol. 2008;325:383-95. doi: 10.1007/978-3-540-77349-8_21. Curr Top Microbiol Immunol. 2008. PMID: 18637517 Review.
Cited by
-
Human cytomegalovirus infection dysregulates the canonical Wnt/β-catenin signaling pathway.PLoS Pathog. 2012;8(10):e1002959. doi: 10.1371/journal.ppat.1002959. Epub 2012 Oct 11. PLoS Pathog. 2012. PMID: 23071438 Free PMC article.
-
High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination.J Virol. 2015 Aug 1;89(15):7673-7695. doi: 10.1128/JVI.00578-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972543 Free PMC article.
-
Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection.Am J Pathol. 2010 Sep;177(3):1298-310. doi: 10.2353/ajpath.2010.091210. Epub 2010 Jul 22. Am J Pathol. 2010. PMID: 20651234 Free PMC article.
-
Cytomegalovirus infection and antibody protection of the developing placenta.Clin Infect Dis. 2013 Dec;57 Suppl 4(Suppl 4):S174-7. doi: 10.1093/cid/cit583. Clin Infect Dis. 2013. PMID: 24257421 Free PMC article.
-
Identification of novel viral interleukin-10 isoforms of human cytomegalovirus AD169.Virus Res. 2008 Feb;131(2):213-23. doi: 10.1016/j.virusres.2007.09.011. Epub 2007 Nov 5. Virus Res. 2008. PMID: 17976852 Free PMC article.
References
-
- Baldanti, F., M. G. Revello, E. Percivalle, N. Labo, and G. Gerna. 2003. Genomes of the endothelial cell-tropic variant and the parental Toledo strain of human cytomegalovirus are highly divergent. J. Med. Virol. 69:76-81. - PubMed
-
- Bass, K. E., H. Li, S. P. Hawkes, E. Howard, E. Bullen, T. K. Vu, M. McMaster, M. Janatpour, and S. J. Fisher. 1997. Tissue inhibitor of metalloproteinase-3 expression is upregulated during human cytotrophoblast invasion in vitro. Dev. Genet. 21:61-67. - PubMed
-
- Britt, W. J. 1999. Congenital cytomegalovirus infection, p. 269-281. In P. J. Hitchcock, H. T. MacKay, and J. N. Wasserheit (ed.), Sexually transmitted diseases and adverse outcomes of pregnancy. ASM Press, Washington, D.C.
-
- Cattaruzza, M., W. Slodowski, M. Stojakovic, R. Krzesz, and M. Hecker. 2003. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J. Biol. Chem. 278:37874-37880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

